Why d block elements are called outer transition elements? Concisely please.
1 Answer
Nov 2, 2017
Refer to the explanation.
Explanation:
The d-block elements are the outer transition elements as opposed to the f-block, which are the inner transition elements. The inner transition elements are part of group 3, and are the elements with atomic numbers
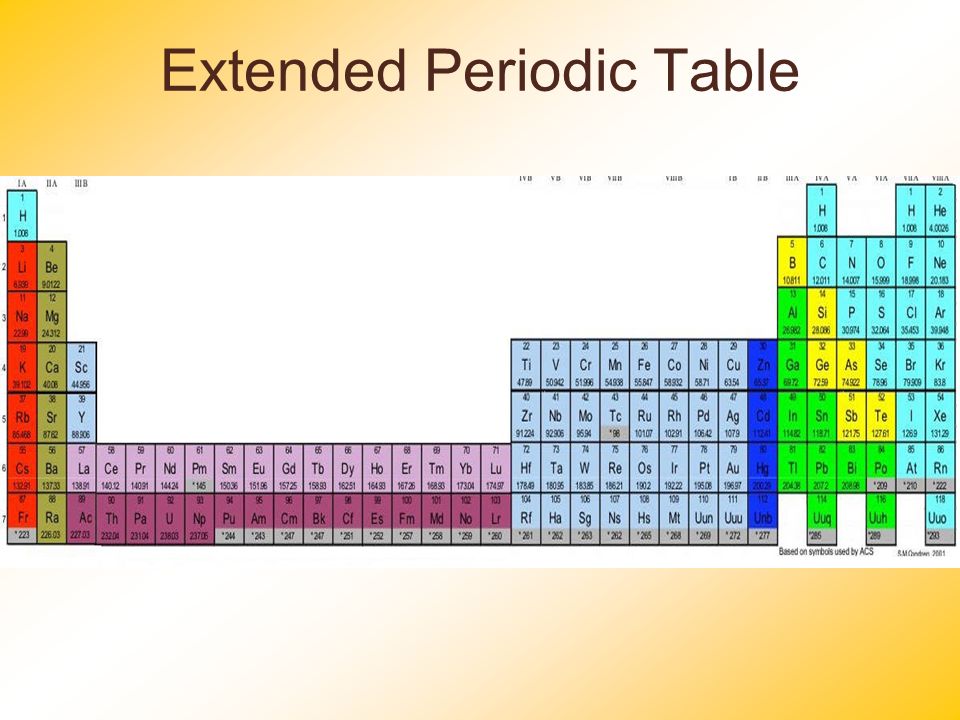
The following picture is an extended periodic table, which includes the inner transition (f-block) elements in two shades of purple. The d-block (light blue) comes after the inner transition elements, so this illustrates why the f-block elements are called the inner transition elements.