What is meant by ionization energy?
1 Answer
Nov 4, 2017
Explanation
Explanation:
Ionization generally means the amount of energy required to steal an electron from an atom. If we try to take out the electron, we will know that low energy is required to pull the valence electron. Whereas, high energy is required to pull the successive electron in an atom.
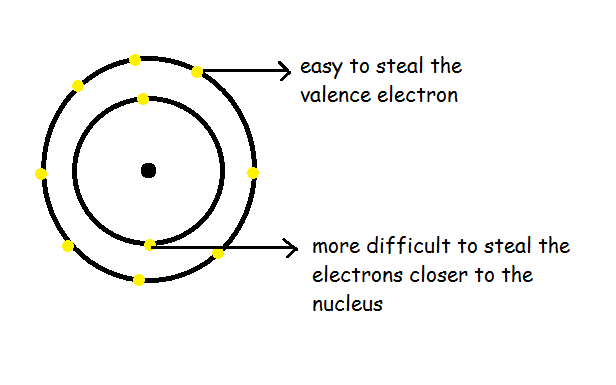
We can notice that the ionization energy of atoms decreases when we go down. But increases when we go in the horizontal direction
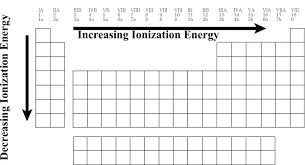
Hope this helps!!! ☺○☻

