What is the order of volatility for the alkane series?
1 Answer
Nov 4, 2017
AS a chemist, as a physical scientist you should interrogate the data...
Explanation:
And, as expected, methane,
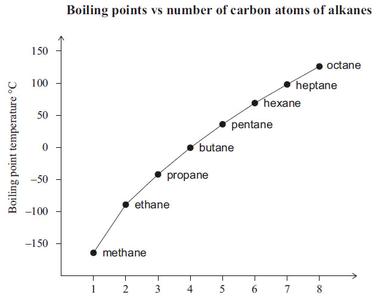
The longer the alkyl chain length, the more opportunity there is for chain-chain interaction by dispersion forces, and thus the MORE involatile the alkane, and the higher the boiling point.

