Question #da3e6
1 Answer
Nov 5, 2017
0.275 M
Explanation:
Using the ICE (initial, change, equilibrium) table
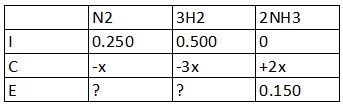
In the Change row,
N2 and 3H2 are negative because they are consumed
NH3 is positive because it is formed
NH3:
0 + 2x = 0.150
x = 0.075 M
Calculating for the amounts in equilibrium,
N2:
0.250 - x
= 0.250 - 0.075
= 0.175 M
H2:
0.500 - 3x
= 0.500 - 3(0.075)
= 0.275 M

