Question #9f08d
1 Answer
Nov 12, 2017
Here's what I get.
Explanation:
An angular node is a planar or conical surface.
A radial node is a spherical surface surrounding the nucleus.
For a given orbital:
#"number of nodes" = n-1# #"number of angular nodes" = l# #"number of radial nodes" = n – l – 1#
For a
#"Number of nodes" = 4-1= 3# #"Number of angular nodes" = 1# #"Number of radial nodes" = 4 – 1 – 1 = 2#
Thus, a
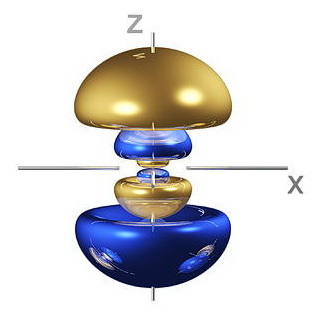
(Adapted from fineartamerica.com)
For example, a
For a
#"Number of nodes" = 5-1= 4# #"Number of angular nodes" = 3# #"Number of radial nodes" = 5 – 3 – 1 = 1#
A
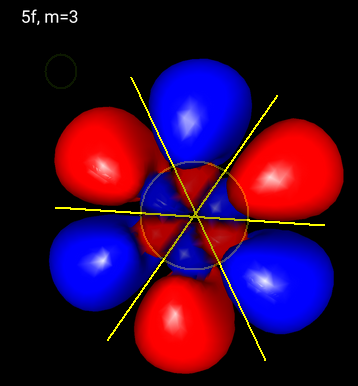
Above is a picture of one of the
You can see a radial node and three planar nodes perpendicular to the screen.

