How would you draw a flow sheet illustrating the seperation and purification of the individual components of a mixture of bezoic acid, aniline, napthalene and p-chlorophenol?
1 Answer
Nov 13, 2017
Here's a chart similar to the one you need.
Explanation:
It uses a mixture of benzoic acid, aniline, anthracene, and phenol.
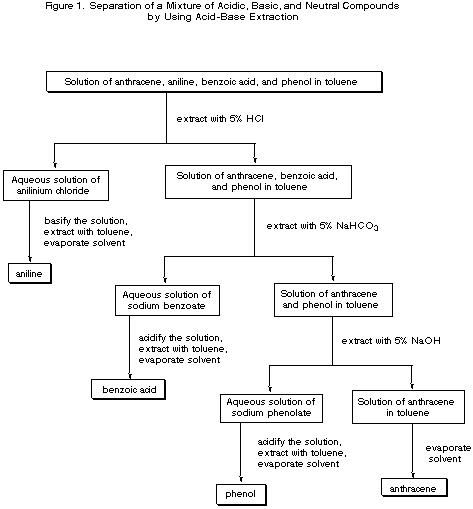
You are separating a base, a neutral compound, a medium strength acid, and a very weak acid.
It is based the following principles:
-
The aniline is basic, so it is removed by
#"HCl"# and then recovered. -
Benzoic acid is a much stronger acid than phenol. Both react with
#"NaOH"# , but only benzoic acid is acidic enough to react with the weaker base#"NaHCO"_3# . The benzoic acid is removed first with#"NaHCO"_3# , followed by removal of the phenol with#"NaOH"# . -
Finally, the neutral aromatic hydrocarbon is removed by boiling off the solvent.
If required, each compound can then be purified by the appropriate methods.

