Why is phenolphthalein a good indicator for acid-base titrations?
2 Answers
Nov 16, 2017
The phenolphthalein will change colour .
Explanation:
As NaOH (Sodium hydroxide) is a alkaline substance so,it changes colour from colourless to PINK .
Nov 16, 2017
The equation is
Explanation:
Phenolphthalein is a weak acid that has different colours in its acid and base forms.
I have indicated the acidic proton by a red arrow.
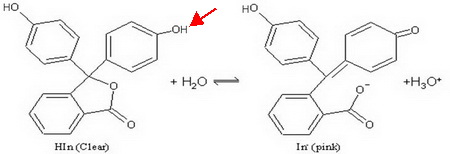
(Adapted from slideplayer.com)
Because it is an indicator, we often write its equilibrium as
That's why it is an excellent indicator for strong acid-strong base titrations.
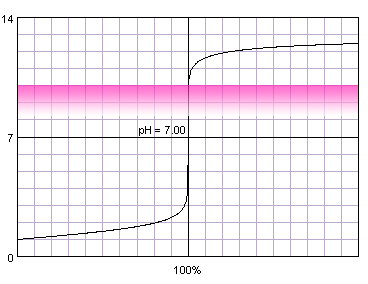
The reaction with


