Why does a water molecule have a bent shape?
1 Answer
Nov 18, 2017
Refer to the explanation.
Explanation:
In the Lewis structure for
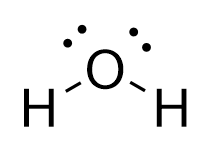
Refer to the explanation.
In the Lewis structure for
