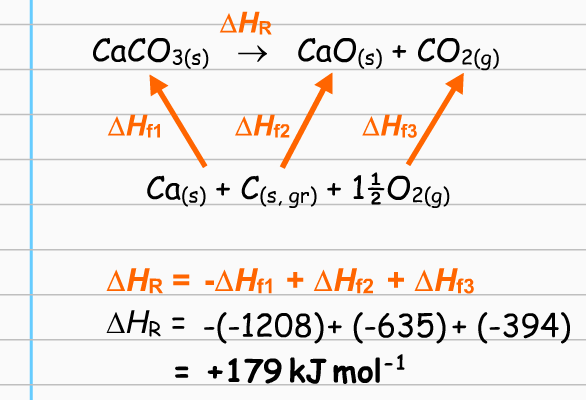
Can someone please explain this diagram to me? How do we know that the arrows are supposed to point upwards and not downwards? Why is O2 and C's arrow pointing to the pointing to the products side?
1 Answer
I think you may be trying to read too much into the diagram. See below...
Explanation:
This problem is looking at the way it is possible to calculate the enthalpy change of a reaction (
The theory behind it is a bit lengthy to explain here, but it comes down to assigning the value of zero to the bond energy of every element (in its natural "elementary" form), and then considering the enthalpy change involved in creating that compound from the elements contained in it.
So, the line below the upward arrows is just a list of the elements found in the compounds of the equation (the "+" signs between the elements means nothing, really).
Then, we are to understand that to form
Likewise for the products,
The upward arrows are just a symbol to show that these enthalpies can be looked up, and applied to the equation. They have no other "directional" meaning (not like a vector, for instance). In particular, it is not meant that one arrow "belongs" to
The bottom equation finishes the job by showing that the actual enthalpy of the reaction,

