Question #20e2a
2 Answers
It is a somewhat arbitrary designation, but meant to indicate which electrode has lower (reduction) potential (the negative anode) and which has a higher value (the cathode). More below...
Explanation:
If you are trying to connect the terminal signs to the reduction potentials of the half-reactions involved at these terminals, then the anode is always the terminal with the lower reduction potential (but not necessarily a negative value!).
With this designation, electrons will be seen to move from anode to cathode (which is always the case, whether you are considering electrochemical cells or electrolytic cells), and also from low potential to higher potential, which fits in properly with the way that physics describes movement of charges, and the work they can do.
The anode contains an oxidation reaction, from which electrons are spawned.
The cathode contains a reduction reaction, which absorbs those electrons
Explanation:
The way a galvanic cell works is that you have a redox (reduction-oxidation) reaction going on, but you separate each component, which means that the electrons from the oxidation reaction need to travel through some path before they reach the reduction component. As they travel through said path, we can harness them for energy.
Quick Reminder: What are Oxidation & Reduction Reactions:
- Oxidation reactions are where electrons are lost (i.e. in the products of the reaction).
- Reduction reactions are where electrons are gained (i.e. in the reactants of the reaction)
Redox Reactions have both of these happening at once.
Take a look at this example of a galvanic cell: 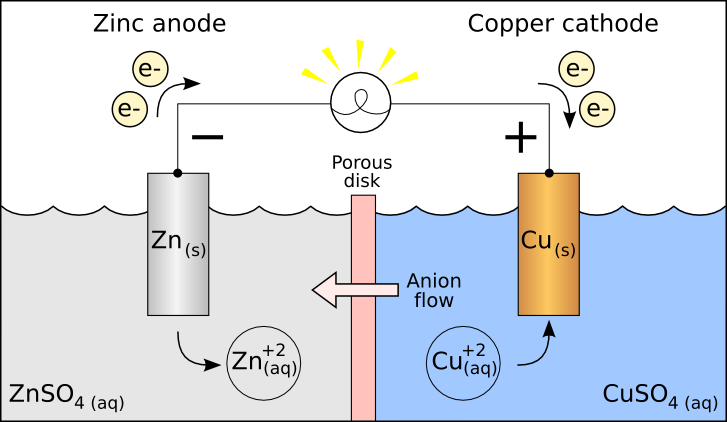
Can you identify where the oxidation and reduction reactions are?
The reason you have the negative on the anode (where the oxidation occurs) is because that's where your electrons are generated.
The reason you have the positive on the cathode (where the reduction occurs) is because that's where your electrons are consumed.
An easy way to remember is that electrons always go from negative to positive in a galvanic cell.
Hope that helped :)


