Question #9f15c
1 Answer
Nov 18, 2017
2 electrons
Explanation:
In an atomic orbital both the electrons have same
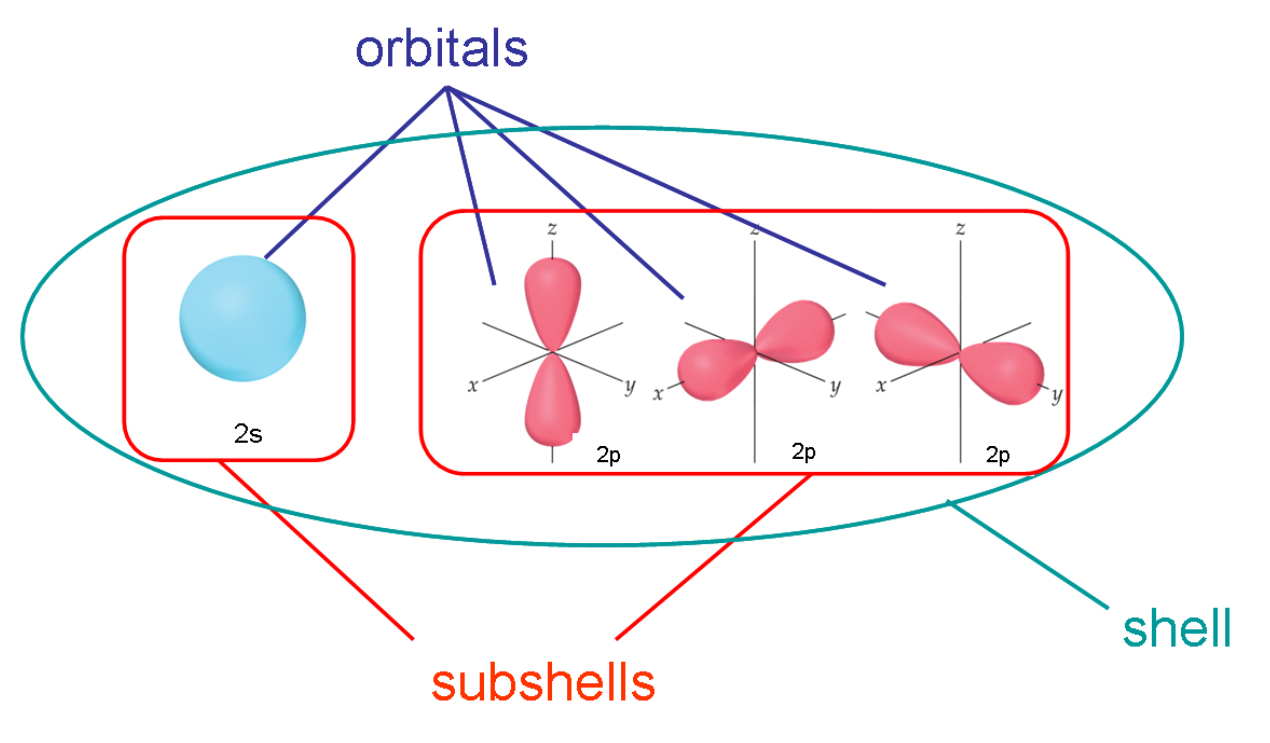
To understand this you should know what is orbit (shell), orbital and sub shell. Below image will help you.