Question #c76d8
1 Answer
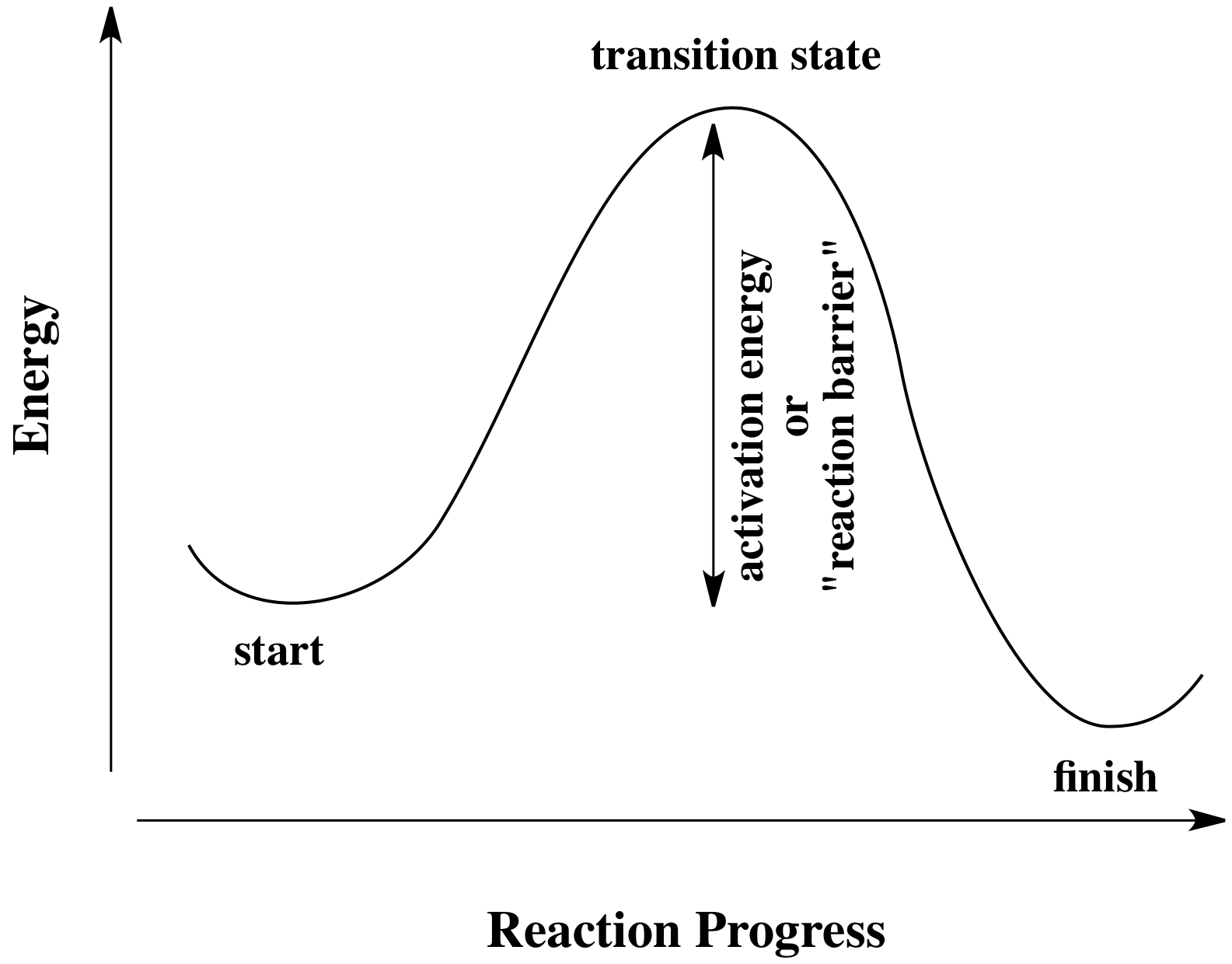
Activation Energy
Explanation:
The minimum amount energy that the reactants need to posses in order to undergo a chemical reaction is called the activation energy.
A more practical example would be climbing a tall mountain. After reaching the peak, then it's all beautiful views and downhill from there.
How that translate into your question is that the energy you need to get to the top of the mountain is called the activation energy.
The very peak is called the Transition State, and from there, the reaction occurs. Now keep in mind that unless someone has enough energy to climb that mountain in my example, they are not going to enjoy the view and the fun downhill portion of it. In the same way, if the molecules do not have enough activation energy to reach the transition state, they will not undergo all the fun chemical reaction(s).
Hope this helped (c:
