A wave function for hydrogen atom is given below, where #a_0# is the Bohr radius, #"52.9177 pm"#. If the Radial node in 2s be at #r_0#. Then the value of #r_0# in terms of #a_0# is?
Here is the equation:
#ψ_(2s) = color(red)(sqrt(2)/(8pi)) × [1/a_0]^"3/2" [2 - r/a_0]e^(-r//color(red)(a_0))#
-
#r_0 = 1/2a_0#
-
#r_0 = 2a_0#
-
#r_0 = 4a_0#
-
#r_0 = 2/sqrt(2)a_0#
Here is the equation:
-
#r_0 = 1/2a_0# -
#r_0 = 2a_0# -
#r_0 = 4a_0# -
#r_0 = 2/sqrt(2)a_0#
1 Answer
#r_0 = 2a_0#
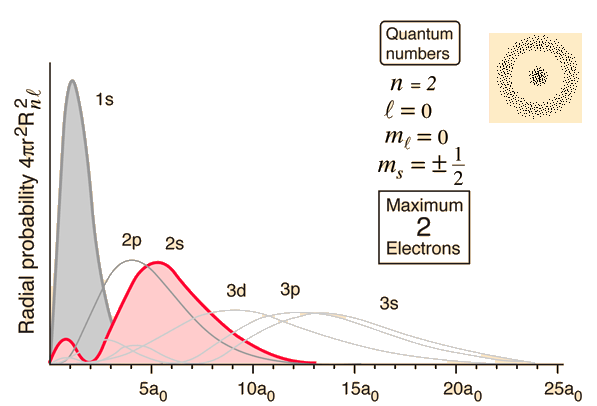
First of all, the wave function you have is not correct. The correct wave function is:
#psi_(2s) = color(orange)(1/(4sqrt(2pi))) (1/a_0)^(3//2)(2 - r/a_0)e^(-r//color(orange)(2a_0))#
The radial node occurs where the radial component
All the nonzero constants can be divided out to get:
#0 = |[(2 - r/a_0)e^(-r//2a_0)]|_(r = r_0)#
Since
#0 = 2 - r_0/a_0#
Therefore, the distance away from the nucleus at which there is a radial node is:
#color(blue)(r_0 = 2a_0)#
or about

