Question #27764
1 Answer
Nov 23, 2017
No.
Explanation:
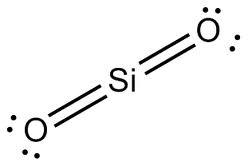 https://www.studyblue.com/notes/note/n/chemistry-acs-review/deck/6237867
https://www.studyblue.com/notes/note/n/chemistry-acs-review/deck/6237867
As you can see from its Lewis structure above, there are no other places for the double bonds between Silicon and Oxygen to go.
A molecule that does have resonance structures, however, is
http://apchemrev.wikispaces.com/Valence+Bond+Theory-+orbitals%3B+resonance%3B+sigma+and+pi+bonds

