Question #a0b96
1 Answer
Nov 25, 2017
How well the acid's ions dissociate in water.
Explanation:
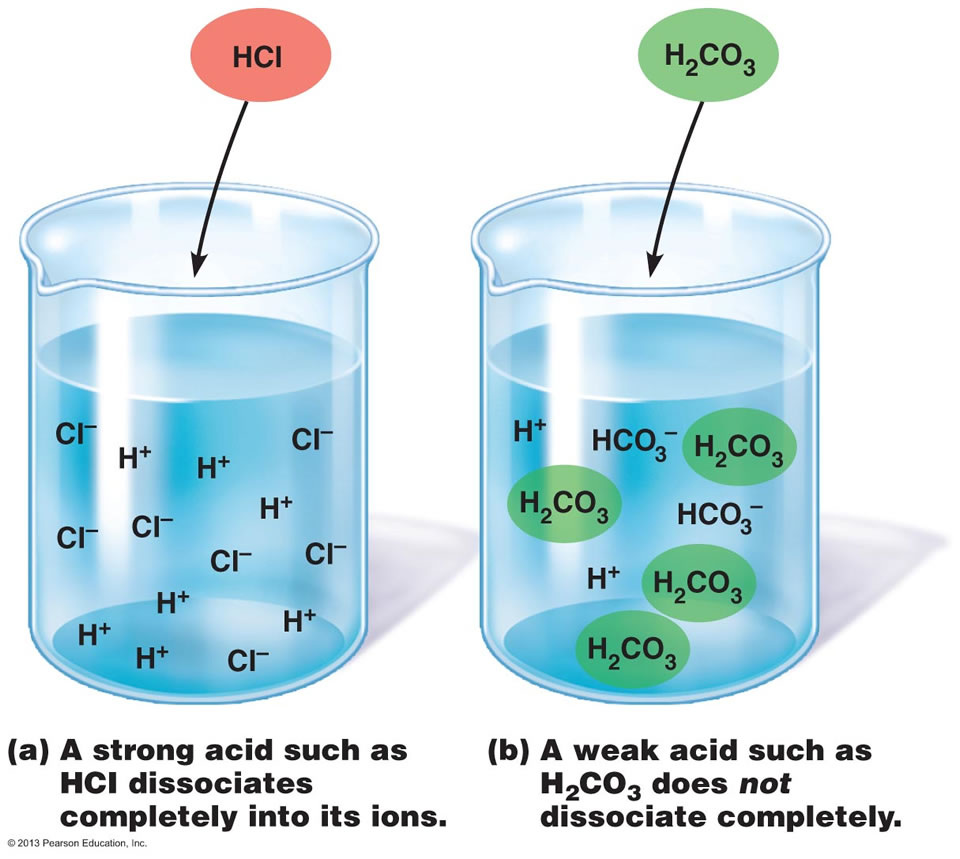
Acids are a class of electrolytes that are classified as being either strong or weak.
Strong electrolytes are ones that dissociate completely into it's ions when dissolved, as seen in the picture below. What this means is (in, for example,
Weak electrolytes are just the opposite: they don't dissociate completely when dissolved in water and some ions still exist in their compounds.