#"200 mL"# of carbon dioxide has a mass of #"0.392 g"#. What is its density?
1 Answer
Nov 27, 2017
The density of carbon dioxide is
Explanation:
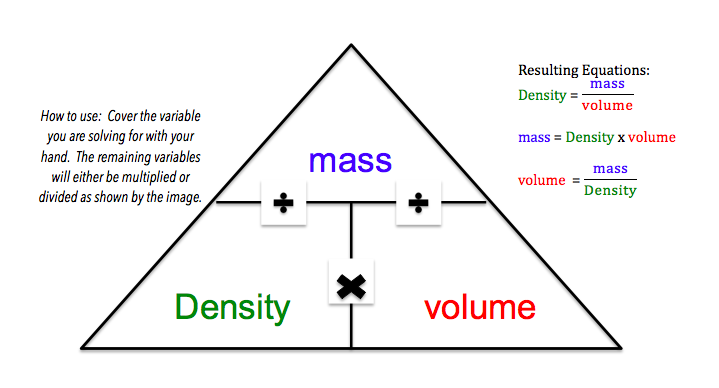
Density is the mass per unit of volume of a substance. The formula for density is:
In this question, the mass of the carbon dioxide
Plug the given mass and volume into the density formula and solve.