When an electron moves from excited to ground state (n=1), why can't it be seen by the human eye?
Because the frequency is too high? Quantum leaps?
And btw please explain this for a highschooler to understand! Thanks
Because the frequency is too high? Quantum leaps?
And btw please explain this for a highschooler to understand! Thanks
1 Answer
This is me just going out on a limb, but I think it's because an electron is simply far too small to reflect enough light for our eyes to sense.
Explanation:
For example, regular household objects are large enough to reflect an adequate amount of light for our eyes to sense. Electrons, however, are too small to reflect an adequate amount of light.
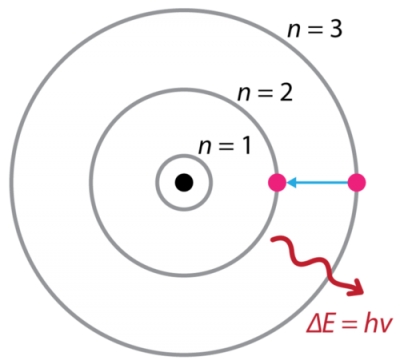
Although we can't see the physical process of an electron actually moving from an excited state to a ground state, we can see the energetic process.
When an electron moves from a higher energy level to a lower energy level, it needs to release energy. This energy is released in the form of light energy, and we can very well see it if it's in the visible light spectrum.
For instance, an electron moving from a n=3 to a n=2 energy level in a hydrogen atom will emit about a 660 nm wavelength of light.
If this is observed in a spectroscope, you will see a distinct red line, corresponding to the 660 nm wave the electron emitted.
Here is a Bohr Model of the electron emitting the light energy: