Question #e9d68
1 Answer
Dec 2, 2017
Explanation:
Atomic number of
Electronic configuration of
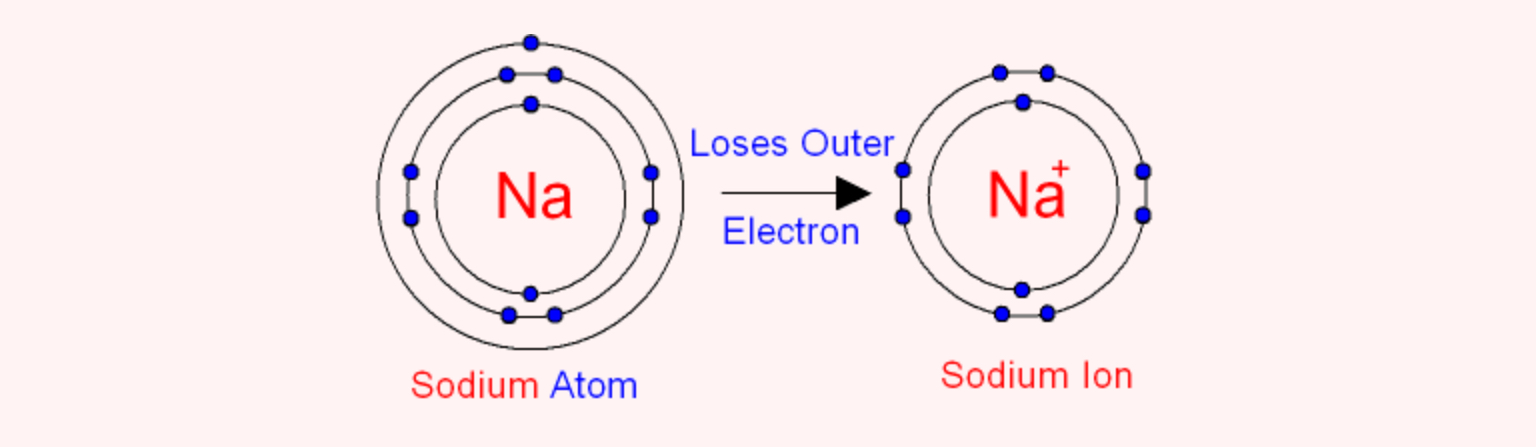
Electronic configuration of
Atomic number of
Electronic configuration of

Electronic configuration of