Question #f7918
1 Answer
Explanation:
The trick here is to recognize that the two products of this potential double-replacement reaction are soluble in water, which implies that exist as ions in aqueous solution.
Since both reactants are soluble in water, they too exist as ions in aqueous solution. This implies that every ion that is present in the solution is a spectator ions.
Consequently, you can say that this double-replacement reaction does not take place.
#"MgI"_ (2(aq)) + "Na"_ 2"SO"_ (4(aq)) -> color(red)("N.R."color(white)(.)("no reaction"))#
So, you know that when you mix solutions of magnesium iodide and sodium sulfate, you get aqueous magnesium sulfate and sodium iodide. The solubility rules for ionic compounds confirm that all four chemical species are indeed soluble in water.
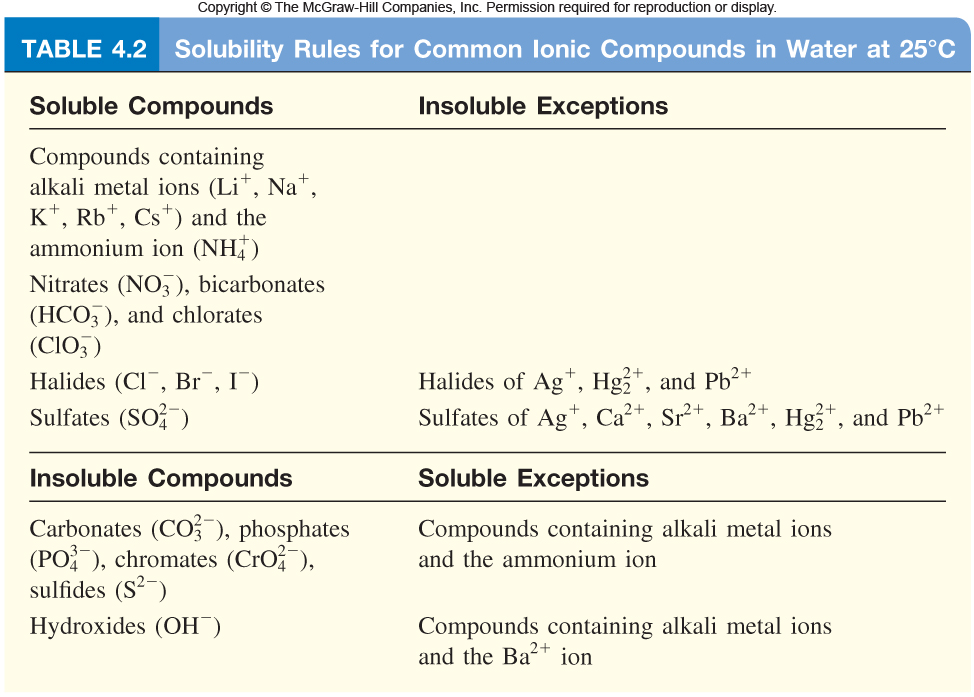
In aqueous solution, you have
#"Mg"_ ((aq))^(2+) + 2"I"_ (2(aq)) + 2"Na"_ ((aq))^(+) + "SO"_ (4(aq))^(2-) -> "Mg"_ ((aq))^(2+) + "SO"_ (4(aq))^(2-) + 2"Na"_ ((aq))^(+) + 2"NO"_ (3(aq))^(-)#

