Calculate #DeltaG_f^°# of #CH_4# from the tabulated values?
What is #DeltaG_f^°# of #CH_4(g)# at #298K# ?
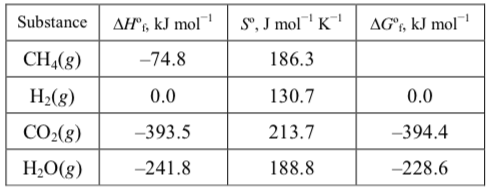
What is

1 Answer
Seemingly simple, this takes a bit of toying around (took me two hours!): we need to construct an equation from the values, as such,
Derive the enthalpy and entropy of the reaction,
Calculate the standard free energy of reaction from here,
and finally, solve for the free energy of formation of methane,

