Question #795c5
1 Answer
Rutherford's 'gold foil experiement'
Explanation:
Rutherford bombarded ultra thing gold foil with alpha particles (positively charged particles consisting of two protons and two neutrons).
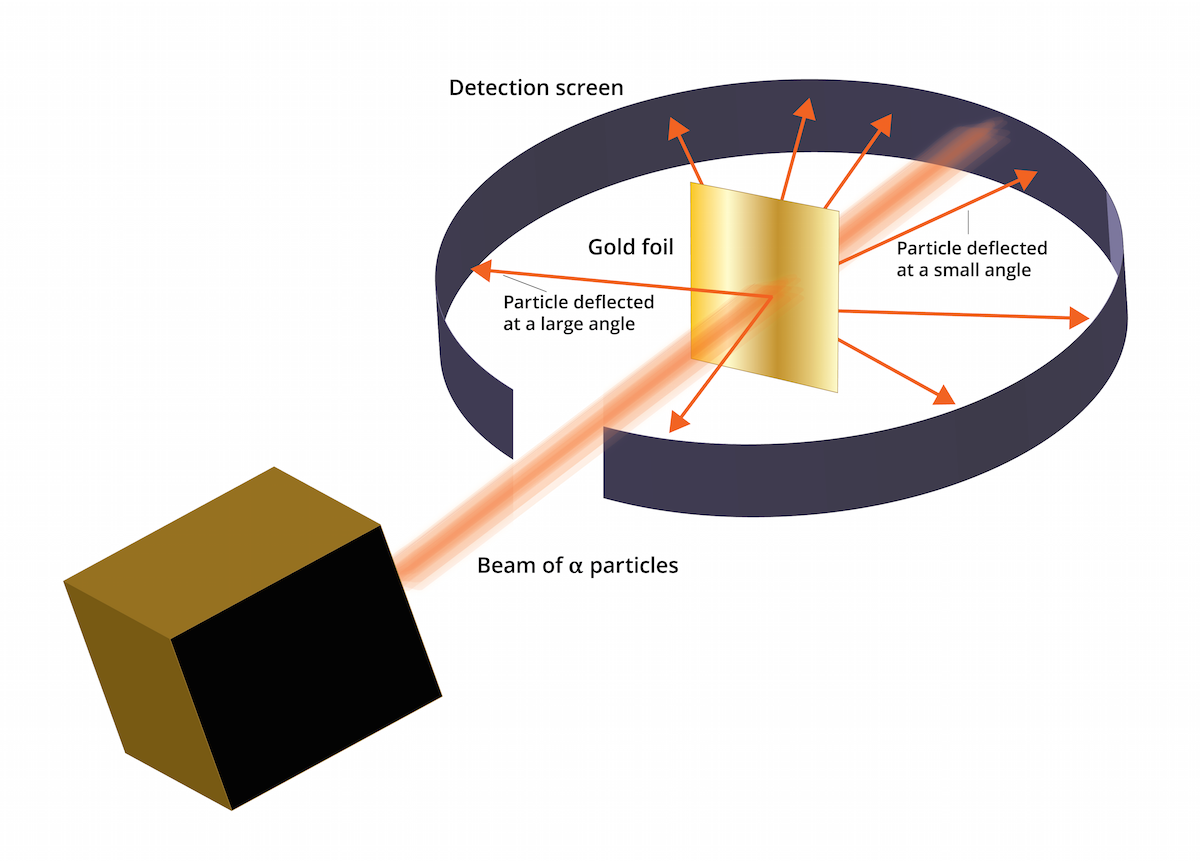
Specific to his experiment was the tracking of these particles - following where they went after hitting the surface of the foil. The vast majority passed straight through the foil in the same direction as they entered - thus implying that the majority of the structure of an atom is empty (or at the very least, neutral) space.
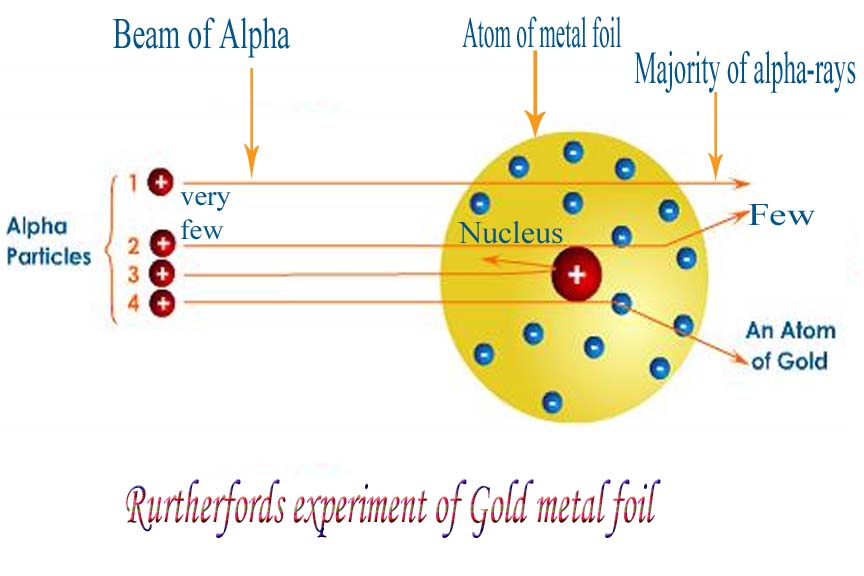
What proved most interesting, however, was that SOME of the particles changed direction and even, in some cases, 'bounced' straight back in the opposite direction. Rutherford's explanation for this occurrence was that a densely charged positive mass was causing the alpha particles to change course (as a result of electrostatic repulsion - remember an alpha particle is positively charged). Two positive charges will repel and attempt to rearrange themselves to avoid this repulsion effect. In the case of an atom, it is unable to move drastically and thus the alpha particle moves to minimise the effect of repulsion between the two.
This densely charged positive mass was described as an atoms 'nucleus' and which we describe today as consisting of positively charged protons and neutral neutrons (thus resulting in a positively charged nucleus).
Rutherford himself described the occurrence of alpha particles bouncing back from the gold foil as being 'like throwing a football at a piece of tissue paper and it bouncing back'. That is how strong the electrostatic repulsion is between the nucleus and alpha particles.

