Question #44455
1 Answer
The process is as simple as it sounds - you just take each volume value and take its reciprocal to be your new x-coordinate.
Explanation:
The process is as simple as it sounds - you just take each volume value and take its reciprocal to be your new x-coordinate. An example will probably help show what to do:
Imagine you have a sealed container with a piston attached, so that you can change the volume and read out the pressure. We start with one liter of gas at atmospheric pressure. If we work in liters for volume and atmospheres for pressure we have a starting point of:
for our
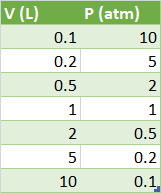
When we graph this we get:
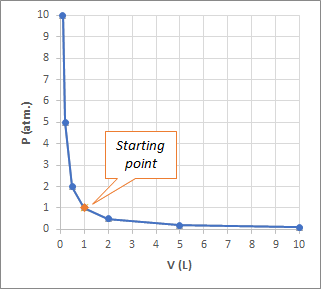
This is the expected behaviour for Boyle's law which states:
where
The trick we are looking for is to "linearize" this equaition by taking our new
This is the equation of a straight line through the origin, which makes it very easy to experimentally determine
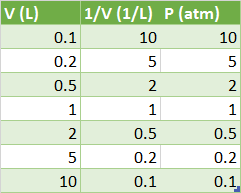
Then when we plot
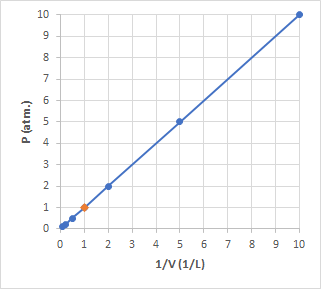
For more information you can look here

