The key idea of "ligand field theory" is that a "ligand field" or "crystal field" imposed by 6 ligands coordinating to a cationic metal centre in an octahedral array, STRONGLY differentiates between the axial "d-orbitals", and the "non-axial orbitals....."
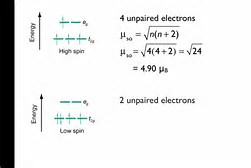
Because the non-axial orbitals are offset from the six vertices of the octahedron, these orbitals, underbrace(d_"xz", d_"yz", d_"xy")_(t_2g" set") are somewhat stabilized with respect to the axial orbitals, underbrace(d_(x^2-y^2),d_(z^2))_(e_g" set")....depending on the electronic configuration, d^4, d^5, d^6, and d^(7) electronic configurations can be either "LOW SPIN (electrons paired)", or "HIGH SPIN (electrons unpaired)", in the resultant transition metal complex. And thus a simple measurement of magnetic moment, can probe the electronic structure of the metal complex.
 youtube.com
youtube.com
Low spin and high spin configurations of a d^4 metal complex are shown. You will have to read your text for the other configurations.....
 youtube.com
youtube.com 
