When a molecule has an #sp^3d# hybridization with 2 lone pairs, the molecular geometry is T-shaped. Why is the T-shape preferable over a trigonal planar shape? (ie, why are the lone pairs positioned that way?)
1 Answer
Dec 20, 2017
Well, as always, it boils down to lone-pair-bonding-pair repulsions being greater in a hypothetical trigonal planar geometry.
With 5 electron groups, a T-shaped molecular geometry would have
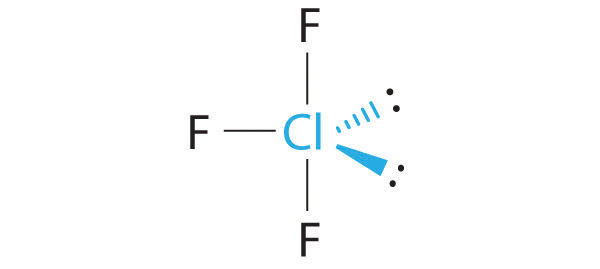
- two
#120^@# interactions (equatorial-equatorial) - one
#90^@# interaction (equatorial-axial)
relative to each lone pair, whereas a hypothetical trigonal planar molecular geometry would have
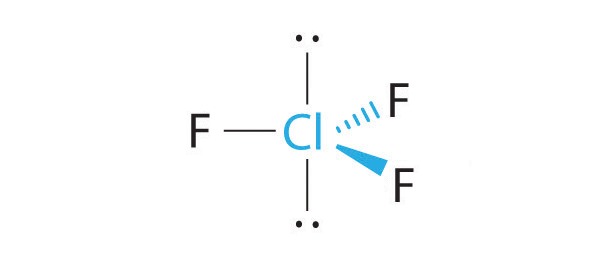
- three
#90^@# interactions (equatorial-axial)
relative to each lone pair.
Thus, the electrons are not maximally-spaced, and the T-shaped molecular geometry for a trigonal bipyramidal electron geometry is favored.

