Well, I can't tell whether you mean #"NO"^(2+)# or #"NO"_2^(+)#... Apparently, maybe you meant #"NO"_2^(+)#. Its bond order is #2#.
If you mean #"NO"^(2+)#, the MO diagram of #"NO"# is:
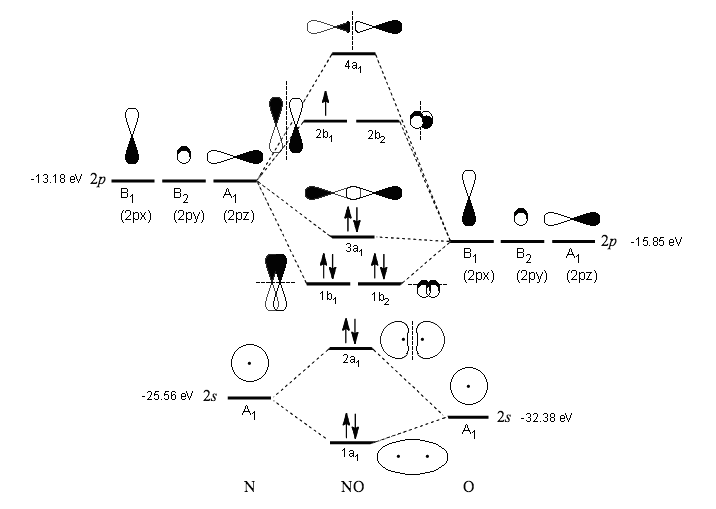
Thus, #"NO"^(2+)# loses the #2b_1# antibonding electron and the #3a_1# bonding electron, and its bond order is around #2.5#. This does not match the given answers, but you had left that possibility open.
If you mean #"NO"_2^(+)#, it is isoelectronic with #"CO"_2#, whose bond order is #bb2#.
How do I know? #"N"# has #7# electrons, and #"C"# has #6#. Thus, swapping out #"C"# for #"N"# and then subtracting one electron to give a #+# charge results in the same number of electrons, which usually results in the same molecular geometry.
Here is #"NO"_2#:
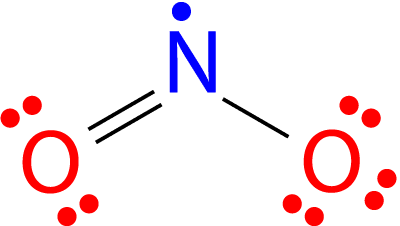
Thus, removing one electron results in a linear geometry, as it convinces the right-hand oxygen to form a bond with the central #"N"# atom:
#:ddot"O"=stackrel((+))("N")=ddot"O":#



