What kinds of speeds can be found from a Maxwell-Boltzmann distribution?
1 Answer
See below.
Explanation:
Concerning the velocity distribution for a Maxwellian gas:
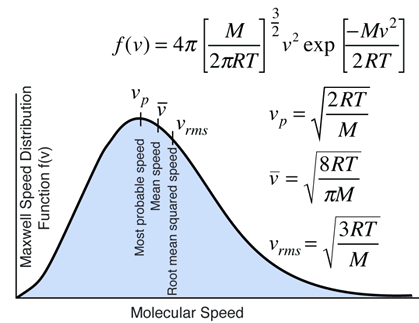
Most probable speed
- The most probable speed corresponds to the maximum of the velocity distribution, where the slope is zero. One solves the equation
(dbar(f)(nu))/(dnu)=sqrt(2/pi)(m/(kT))^(3/2)[2nu+((-mnu)/(kT))nu^2]e^((-mnu^2)//(2kT))=0
where
From this, the most probable speed, denoted
color(blue)(nu_"m.p."=sqrt((2kT)/m))
Mean speed
- An average or mean speed
< nu > is computed by weighting the speednu with its probability of occurrencebar(f)(nu)dnu and then integrating:
< nu > = int_0^(oo)nubar(f)(nu)dnu=int_0^(oo)e^((-mnu^2)//(2kT))sqrt(2/pi)(m/(kT))^(3/2)nu^3dnu
=> color(blue)(< nu > = sqrt(8/pi)sqrt((kT)/m))
Root mean square speed
- A calculation of
< nu^2 > proceeds as:
< nu^2 > = int_0^(oo)nu^2bar(f)(nu)dnu=3(kT)/m
=>1/2m< nu^2 > = 3/2kT
=>color(blue)(nu_"r.m.s"=sqrt((3kT)/m))
Note:
-
The mean speed
< nu > is13% larger thannu_"m.p." andnu_"r.m.s" is22% larger. -
The common proportionality to
sqrt(kT//m) has two immediate implications: higher temperature implies higher speed, and larger mass implies lower speed.
**The equivalent expressions in terms of the universal/ideal gas constant

