What wavelength in #"nm"# corresponds to the limiting line of the Lyman series for hydrogen atom?
1 Answer
I got
Well, you remember limits? You can approach
The Lyman series is in reference to
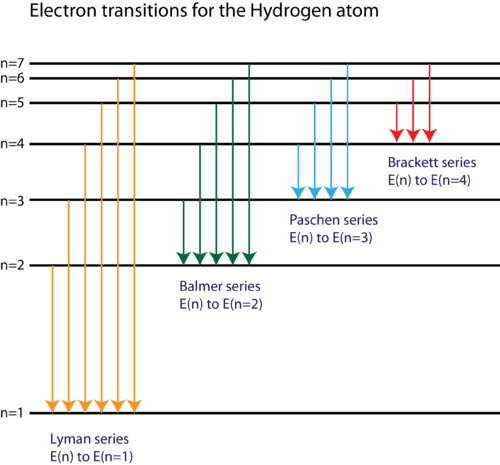
Any emission line in hydrogen atom will involve a change in energy from the Rydberg equation:
#DeltaE = -"13.61 eV" (1/n_f^2 - 1/n_i^2)# where
#n# is as defined before.#f# indicates final and#i# indicates initial states.
The limiting line is simply when
Thus, we expect this to be the ionization energy of hydrogen atom.
#color(green)(|DeltaE|) = lim_(n_i->oo) [|-"13.61 eV"| cdot (1/1^2 - 1/n_i^2)]#
#=# #color(green)("13.61 eV")#
and this is indeed the ionization energy of hydrogen atom.
As a result, we can now find the wavelength needed to ionize hydrogen atom.
#|DeltaE| = E_"photon" = hnu = (hc)/lambda# where
#c = 2.998 xx 10^(8) "m/s"# is the speed of light and#h = 6.626 xx 10^(-34) "J"cdot"s"# is Planck's constant.
Therefore,
#color(blue)(lambda) = (hc)/(|DeltaE|)#
#= (6.626 xx 10^(-34) cancel"J"cdotcancel"s" cdot 2.998 xx 10^(8) "m" cdot cancel("s"^(-1)))/(13.61 cancel"eV" xx (1.602 xx 10^(-19) cancel"J")/(cancel"1 eV"))#
#= 9.11 xx 10^(-8) "m"#
#=# #color(blue)ul("91.1 nm")#

