Transition elements, such as chromium, are likely to have what type of oxidation number?
1 Answer
All of the above! Often,
Your guess is as good as mine. Here are all the possible oxidation states of every non-
It seems like the most common oxidation states are:
+2 for most of the 1st row transition metals.+3 and+4 for most of the 2nd row transition metals.+4 for most of the 3rd row transition metals.
That should make sense, because the orbital energies get closer together as we approach higher values of
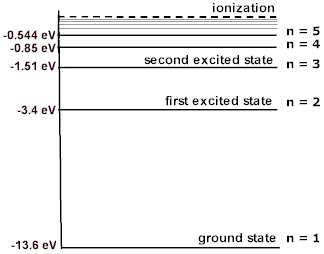
(Although, this convergence is not as patterned in multi-electron atoms, since the angular momentum now present creates non-spherical shapes that complicate the orbital interactions and alter their energies further.)
Still, in general, we can say that for each successive row of transition metals, more orbitals join the valence space, i.e. more electrons take on the role of valence electrons, giving access to higher oxidation states.

