How many structural isomers does butane have?
1 Answer
Jan 2, 2018
Butane has just two structural isomers: i-butane and n-butane.
Explanation:
n-butane, normal butane,
The first one is the normal,unbranched butane. We add the prefix "n-" to the main name "butane".
Lewis structure:
 upload.wikimedia.org
upload.wikimedia.org
Skeletal diagram:
 upload.wikimedia.org
upload.wikimedia.org
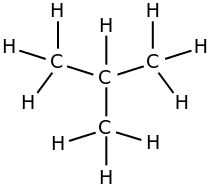
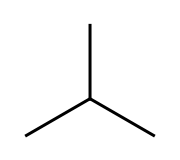
i-butane, isobutane,
The second one is isobutane, "branched" butane. We add the prefix "i-" to the main name. The second name for i-butane is
2-methylpropane
 upload.wikimedia.org
upload.wikimedia.org
 upload.wikimedia.org
upload.wikimedia.org

