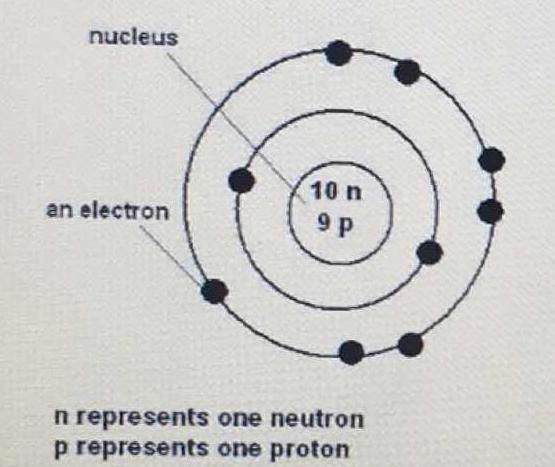
What is the period and group of this atom?
1 Answer
Jan 2, 2018
2nd period and halogen group
Explanation:
First,
Identify the element of this atom.
Since it has 9 protons, and the number of protons corresponds to the atomic number, we look at the periodic table for the element with an atomic number of 9, which is fluorine (F).
Second,
A period in the periodic table means its row. So, fluorine is in the second period.
A group or family means its column. The 7th column has its own name- the halogens.