Why 4 bonds doesn't exist between carbon compounds?
1 Answer
Jan 3, 2018
see below.
Explanation:
I interpret you question to be why 4 bonds don't exist between carbon atoms if my interpretation is wrong , please comment below .
If there were 4 bonds between carbon atoms then the compound formed will be
This compound can exist. its structure is given below .
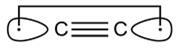 www.royal society of chemistry
www.royal society of chemistry
such a compound is called diatomic carbon .
If you see you can easily make lewis structure of this compound but 4th covalent bond requires overlapping of d-orbital which is absent in carbon .
According to Shaik's report of 2012 this compound can exist but in isolated form , because it is extremely unstable and reactive.

