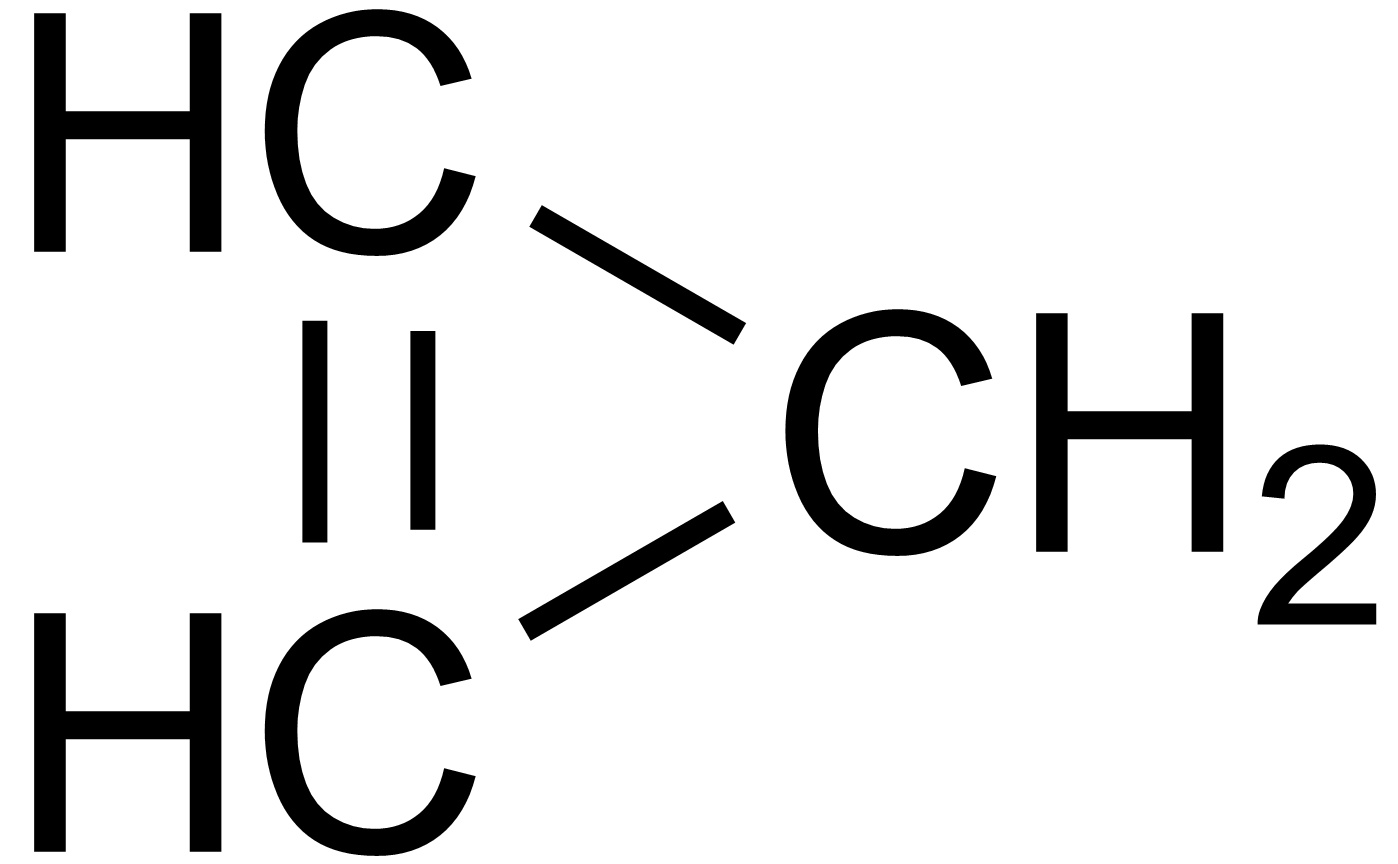How many isomers of #C_3H_4# are possible?
2 Answers
Jan 4, 2018
Well, it could be either.....
Explanation:
You assess the degree of unsatuation.
Each degree of unsaturation corresponds to a double bond, OR a ring junction.....
Jan 4, 2018
There are three structural isomers of
Explanation:
The diagram below represents the structural formula of propadiene.
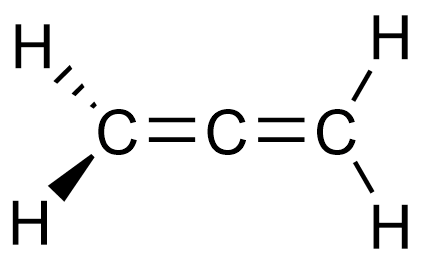
The diagram below represents the structural formula propyne.
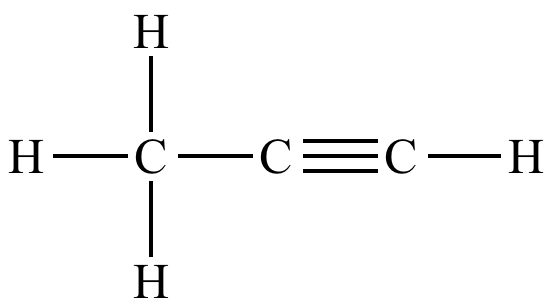
The diagram below represents the structural formula of cyclopropene.