Question #62e5a
1 Answer
Jan 6, 2018
Explanation:
The structure of
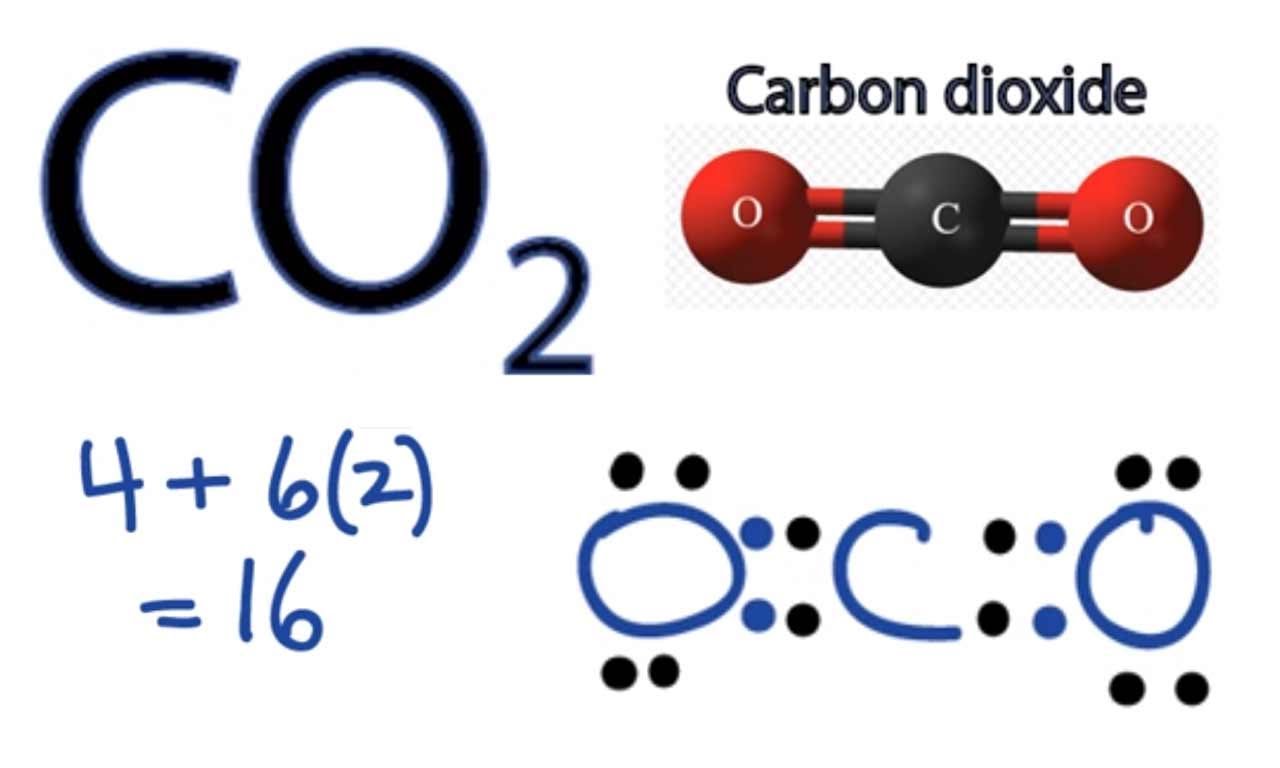
The central atom (carbon) has 8 electrons assigned to it, making it a full octet.
The structure of ICL is
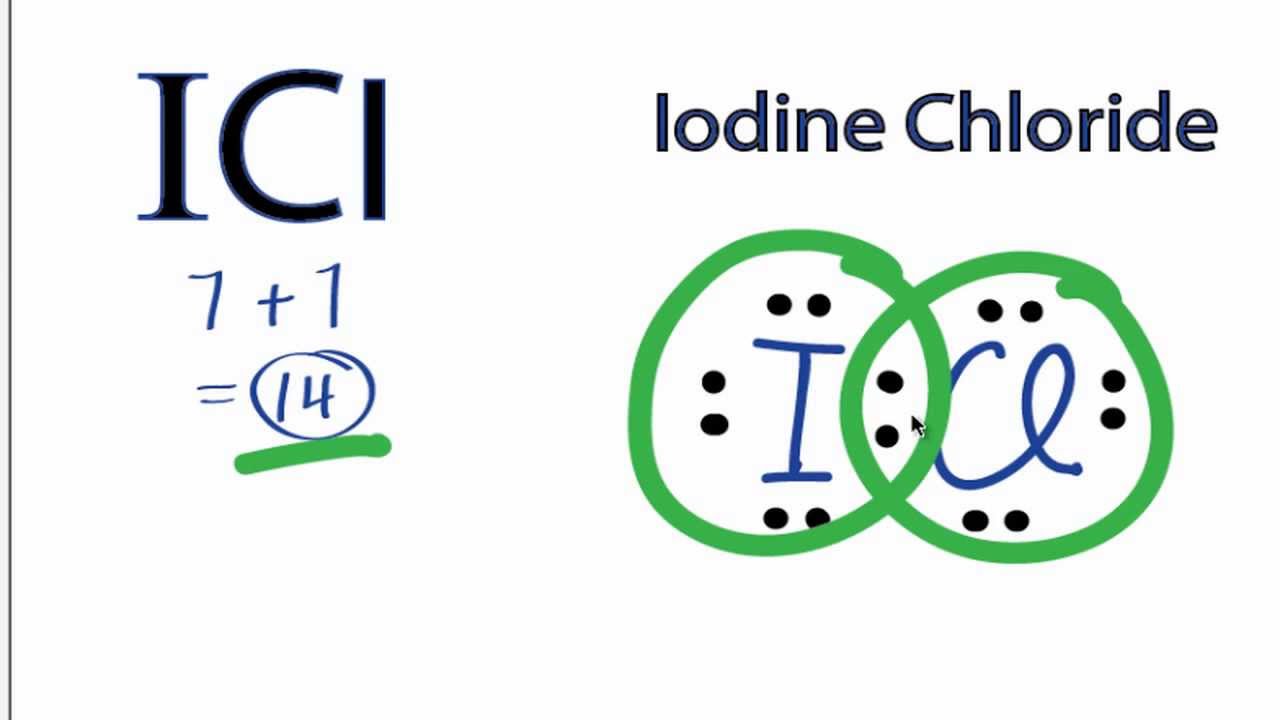
Both iodine and chlorine have 8 electrons assigned to it, making both of them have a full octet.
The structure of
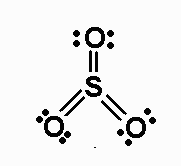
The central atom (sulfur) has a total of 12 electrons assigned to it, so the central atom of
The structure of
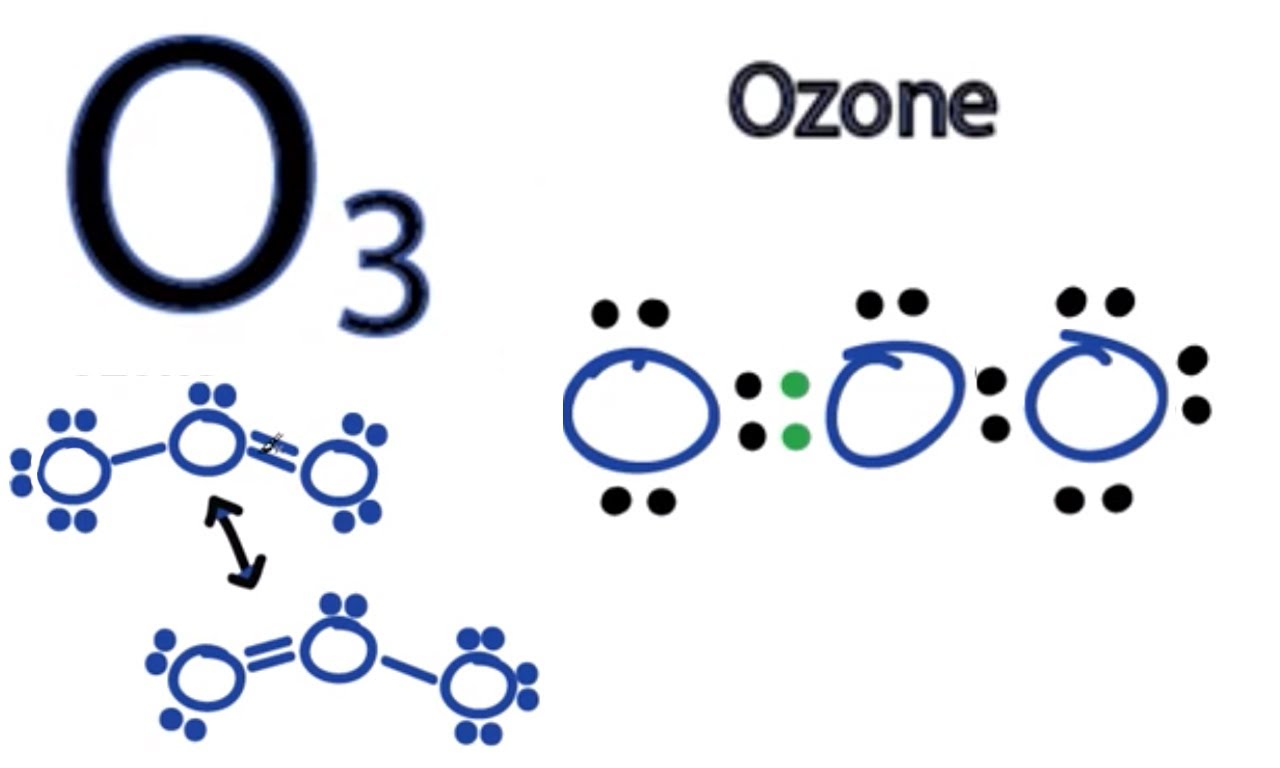
Although

