Question #5f1dd
1 Answer
Atomic mass is the weighted average mass of one atom; molecular mass is the mass of many of these atoms forming a molecule.
Explanation:
Some basic definitions first:
Atomic mass refers to the weighted mass average of an individual atom. (The "average" part of the definition will be explained later.)
Atoms are made of protons, neutrons, and electrons:
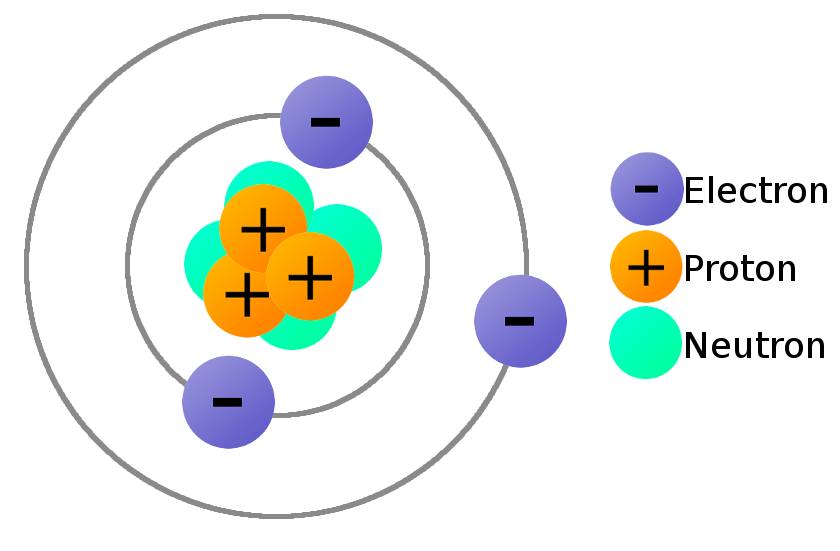
However, we know that not all atoms with the same number of protons, have the same number of neutrons.
These variations of the same element are called isotopes.
A molecule is made when multiple atoms bond together chemically.
Atomic Mass and Molecular Mass:
Atomic mass is the weighted average of all isotopes.
A regular average is:
This treats each item equally.
In this case, however, we can't treat each isotope the same. So, we multiply each isotope by their likelihood of occurrence, in percentages. This basically tells us this: Out of 100 atoms, how many will likely be this isotope?
Here's an example. Given the following isotopes, calculate the atomic mass:
- Mass is 1.008 amu, 99.986% frequency of occurrence.
- Mass is 2.014 amu, 0.014% frequency of occurrence.
We would do this:
- Multiply masses by likelihood of occurrence.
99.986% becomes 0.99986, because
#"1.008 amu"* 99.986 = "100.79 amu"#
#"2.014 amu" * 0.014 = "0.028 amu"# - Take the sum of those masses.
#100.79 + 0.028 = "100.82 amu"# - Divide by the number of items.
We divide by#100# , because this is a percentage.
#"100.82 amu"/100="1.0082amu"#
When calculating molecular mass, we'll probably be provided with a periodic table, which means we won't have to calculate atomic masses.
Here's an example:
There are two hydrogen atoms in water (atomic mass

