Question #05a9c
1 Answer
Explanation:
First, we need to find out how much the
The mass of one water molecule is
- We know water is
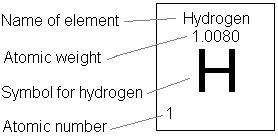
#H_2O# . - By looking at the periodic table, we can find the masses of hydrogen and oxygen—
#"1.008 amu"# and#"16.00 amu"# , respectively.
(The unit used is#"amu"# because the mass of one atom is too small to be dealt with in terms of grams.) #"mass of 2 hydrogen atoms + mass of 1 oxygen atom"#
#"= mass of 1 water molecule"# #(2*"1.008 amu") + "16.00 amu" = "18.02 amu"#
The equation at the beginning can now be applied:
As the mass of the drop of water was given in grams,
Then, we just divide

