What is the hybridization of benzene?
1 Answer
Assuming you mean carbon atom...
...then it is
Benzene is a planar aromatic ring, and has many representations:
Regardless of whether we draw the Kekulé structure or the delocalized representation, the structure is a ring containing carbon atoms that each had formed their first bond in three directions.
It's just like compounds such as
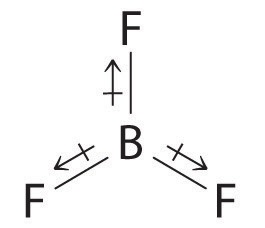
The first bond made by an atom is preferentially a

