The #2p_z# orbital, i.e. the #2p# orbital pointing directly along the internuclear (#z#) axis.
The #bbsigma#/sigma bond is always made along the internuclear axis, overlapping the orbitals head-on. By convention, for linear molecules, that is the #z# axis.
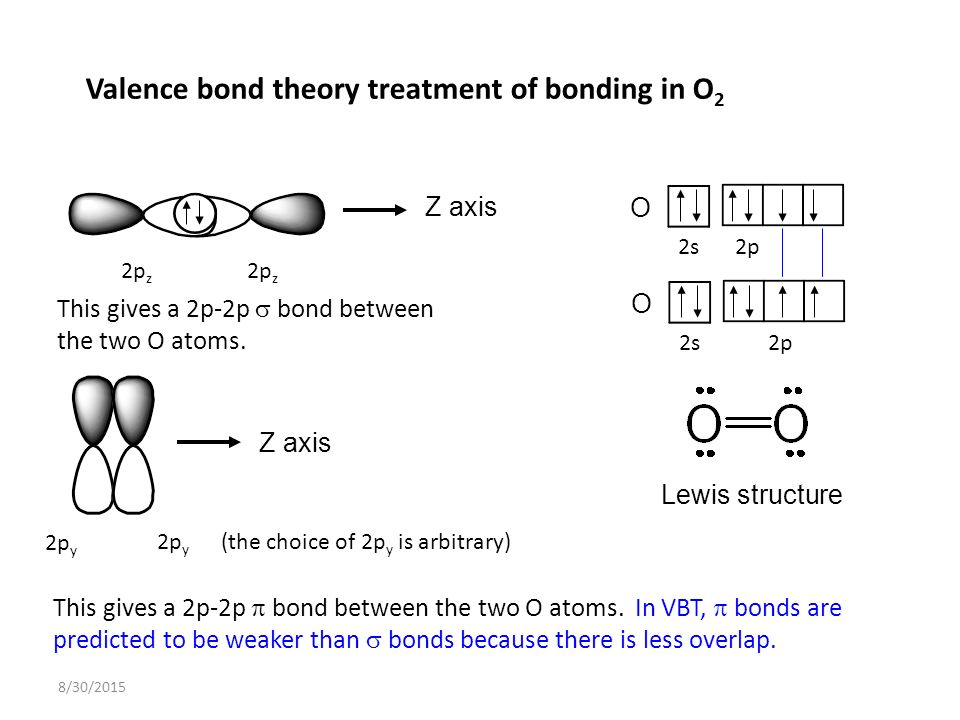
On the other hand, the one #pi# bond in #"O"_2# is made using either a #2p_x# or #2p_y# orbital (arbitrary). For #"O"_2#, there does NOT have to be hybridization, either.
See here for "when" to hybridize.

