What are the balanced thermochemical equations for the dissolving of calcium chloride In water?
2 Answers
Start by writing out your reactants:
Synthesis reaction? No, you can't have
Decomposition reaction? No, there are two reactants.
Combustion reaction? No, there's no carbon.
Single replacement? No, there are not 3 elements in total.
Double replacement? Seems like it; there are 4 elements making up two compounds.
We now predict products:
Warning! Long Answer. The balanced equation is
Explanation:
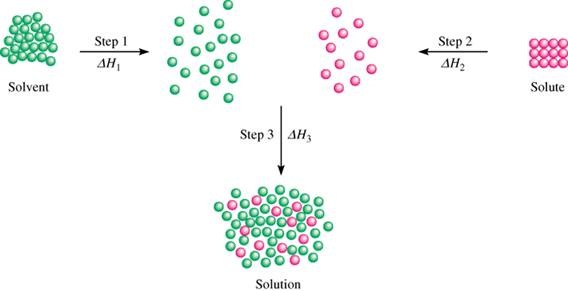
There are three steps in the process of dissolving
Step 1. The ions must separate from each other
It takes energy to separate the ions from each other, so this step is endothermic.
Step 2. The water molecules must separate from each other to make room for the ions
It takes energy to separate the water molecules from each other, so this step is also endothermic.
Step 3. The ions interact with the water molecules and form hydrated ions.
Some of the water becomes water of hydration.
This step is exothermic, so
The overall process
The overall process is the sum of the three steps.
When calcium chloride dissolves, the energy released by hydrating the ions
(
Thus, the dissolving of calcium chloride in water is an exothermic process, and the heat evolved goes into heating the solution in the heating pack


