In the electrolysis of water...?
a) Is hydrogen collected at the cathode or anode? Oxygen?
b) How does the volume of hydrogen made compare to the volume of oxygen made?
c) Why does adding #"H"_2"SO"_4# help?
a) Is hydrogen collected at the cathode or anode? Oxygen?
b) How does the volume of hydrogen made compare to the volume of oxygen made?
c) Why does adding
1 Answer
A. Hydrogen is collected at cathode and oxygen is collected at anode.
Explanation:
In water,
The structure of water is given below
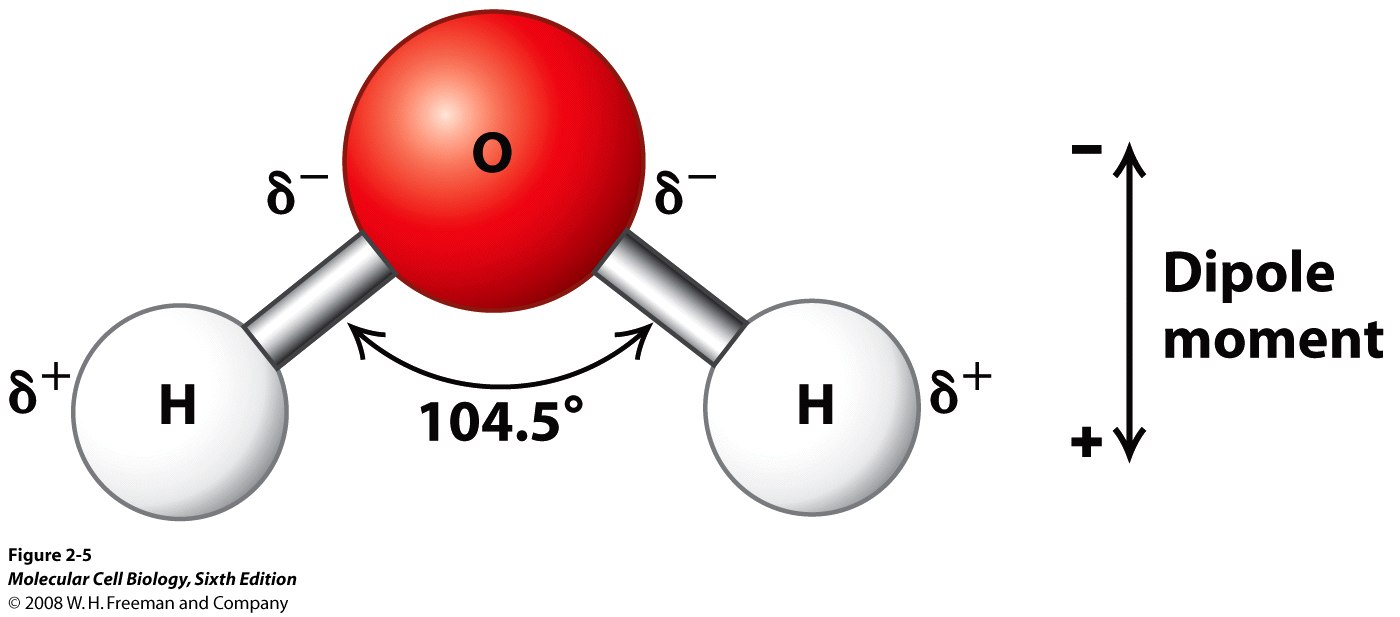
Electronegativity is the measure that how much an atom can attract the shared pair of electrons in a covalent bond.
Opposite charges attract each other while like charges repel each other.
As a result during electrolysis , the negative part that is oxygen is attracted towards the positive electrode, i.e. the anode, while the positive part, that is hydrogen, is attracted towards the negative electrode, i.e. the cathode.
B. At cathode the volume of the gas
Thus, the volume of hydrogen that is collected at the cathode is double the volume of oxygen that is collected at anode.
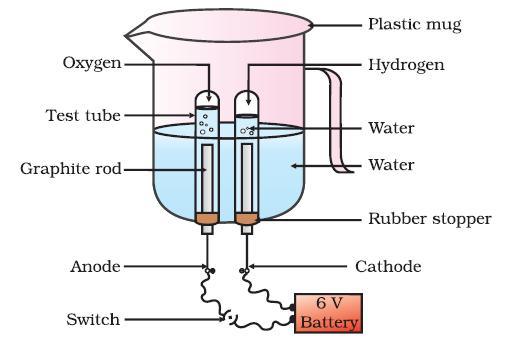
You can see in the figure , the volume of hydrogen gas evolved at the cathode is double to the volume of oxygen gas evolved at the anode.
C.
Thus,


