Question #f2df8
1 Answer
Jan 14, 2018
(The rest of the answers are in explanation.)
Explanation:
To name a covalent binary compound, add -ide to the second element and add numerical prefixes to both elements.
If there is only one atom of the first element, however, don't add mono- to it.
For a more in-depth tutorial on naming, this answer is awesome!
Here's a table of common numerical prefixes:
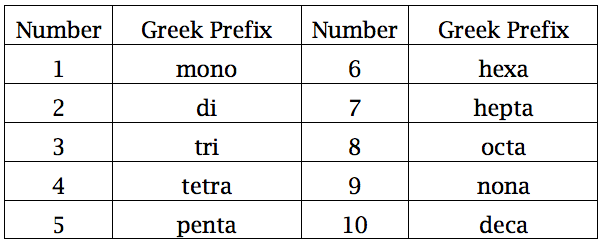
#SiCl_4# is silicon tetrachloride.#SCl_4# is sulfur tetrachloride.#NCl_3# is nitrogen trichloride.#P_2O_3# is diphosphorus trioxide.#SCl_6# sulfur hexachloride.#NF_3# is nitrogen trifluoride.#SF_4# is sulfur tetrafluoride.#P_2S_5# is diphosphorus pentasulfide.#PBr_5# is phosphorus pentabromide.#N_2O_5# is dinitrogen pentoxide. (Not usually penta-oxide, because it sounds a bit weird.)#As_2O_3# is diarsenic trioxide.#B_2O_3# is diboron trioxide.#OF_2# is oxygen difluoride.#SeBr_2# is selenium dibromide.

