Use the half-reaction method to balance the following redox reaction in acidic solution: As2O3(s) + NO3(aq)- ---> H3AsO4(aq) + N2O3(aq) How do you balance a redox reaction in acidic conditions?
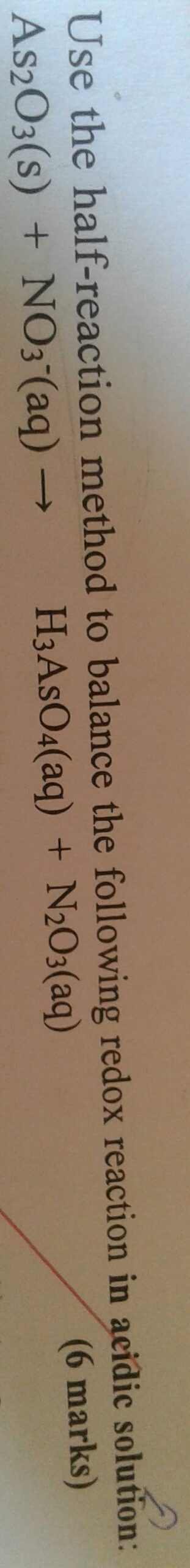
1 Answer
Well you gots nitrate reduced to
Explanation:
...i.e. we gots
And meanwhile arsenic(III) oxide is oxidized to arsenate....
And so we adds the former to the letter to remove the electrons as virtual particles....
...to give...
Please don't assume that I have done my sums right.....And note that when I do a redox equation I ALWAYS assume acidic conditions. If basic conditions are required I would simply add

