What are the constitutional isomers of dimethyl cyclopentane?
2 Answers
Simply saying dimethyl cyclopentane doesn't actually specify which carbon atoms the methyl groups are attached to. As such, it has constitutional isomers, i.e. same number and identity of atoms, different connectivity.
We have three choices. 1,1-dimethylcyclopentane, 1,2-dimethylcyclopentane, or 1,3-dimethylcyclopentane.
(Note how we cannot choose straight-chained hydrocarbons to make cyclopentanes. Also, since you specify "dimethyl", we can only move around methyl groups. Therefore, 1-ethylcyclopentane is not a choice.)
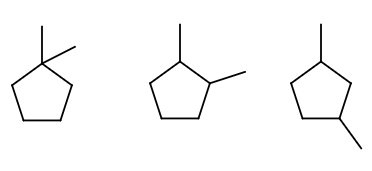
You should convince yourself that any other choice is identical to these three.
- There is no other way to choose 1,1-dimethyl while also minimizing the carbon index.
- Choosing 1,4-dimethyl is just giving you 1,3-dimethyl going the other way by rotational symmetry.
- Choosing 1,5-dimethyl is just giving you 1,2-dimethyl going the other way by rotational symmetry.
Constitutional isomers are isomers of a molecule that differ in atomic connectivity. These are one of the basic skills of organic chemistry you need to master early on,
Consider 1,2-dimethylcyclopentane,

With the molecular formula,


I'm sure I could draw a few more, but you get the point.
Note: if you're only curious about different methyl arrangements around dimethylcyclopentane, only consider the molecules with pentagons.


