Question #e231f
1 Answer
Jan 17, 2018
Explanation:
The thing to remember here is that the mass number of an atom tells you the number of protons and of neutrons located inside its nucleus.
#color(blue)(ul(color(black)("mass number" quad (A) = "no. of protons" quad (Z) + "no. of neutrons")))#
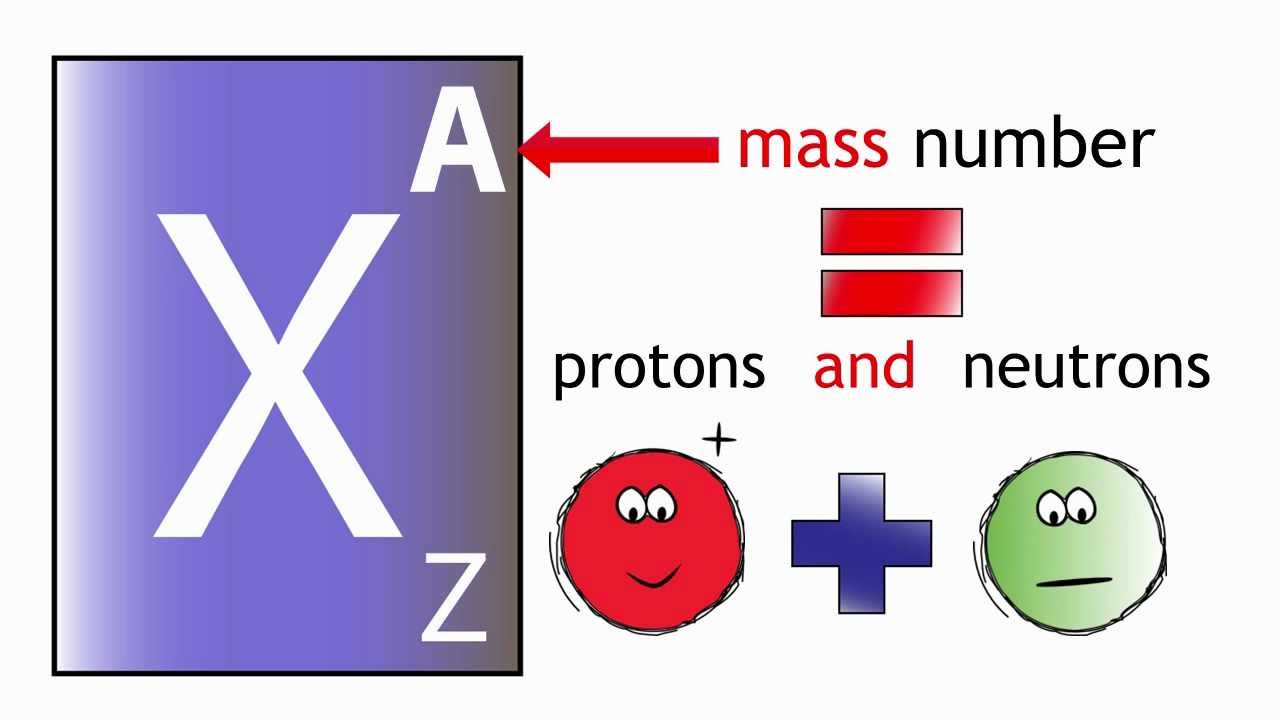
Now, you know that the atomic number tells you the number of protons located inside the nucleus, so you can say that in order to find the number of neutrons located inside the nucleus, you need to subtract the atomic number,
#color(blue)(ul(color(black)("no. of neutrons" = A - Z)))#
This means that your atom contains
#"no. of neutrons" = 32 - 16 = 16#
neutrons inside its nucleus.

