Hydrolysis of the dimer sucrose gives us what result?
2 Answers
Glucose and Fructose....
Explanation:
Hydrolysis of Sucrose will yield Glucose and Fructose, but in Nature this will proceed at a very slow pace, if at all: A sucrose solution is very stable.
Add an enzyme however, (e.g. Sucrase, Invertase ), and there will be a rapid breakdown.
The bond between the two monosaccharides is an acetal bond, and can therefore also be hydrolysed by acids, like lemon juice or gastric juices... You may also know it as a glycosidic bond.
Hydrolysis of the dimer sucrose

Explanation:
Sucrose is a dimer composed of two monomers, glucose and fructose.
Hydrolysis is the degradation of complex covalent chemical compounds in the reaction with water. During hydrolysis the covalent bonds crack, and the hydrogen ion (atom) from the water joins with one product, and the hydroxide ion (hydroxyl group) joins with the another product. In this case below, the hydrogen ion goes with fructose and the hydroxyl group goes with glucose.
Saccharification:
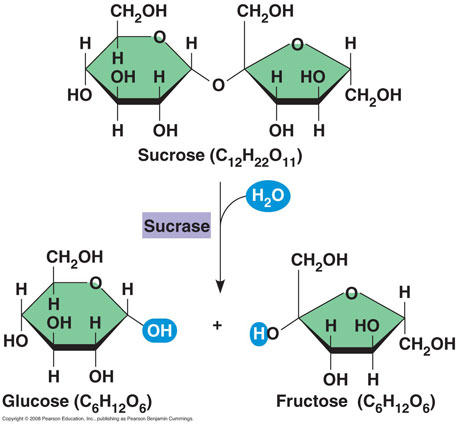
This process of transferring sucrase into glucose and fructose catalyzes the enzyme of invertase and also saccharase. To make the enzymes active, an appropriate acidic medium (pH of 3 to 5) is required.



