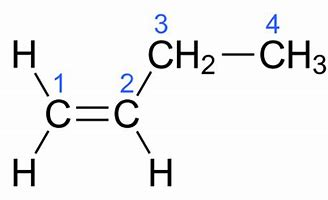
We gots "1-butylene", H_2stackrel1C=stackrel2Cstackrel3CH_2stackrel4CH_3...
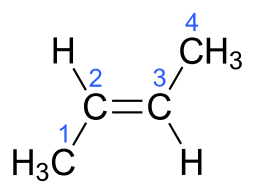
versus "2-butylene", H_3stackrel1Cstackrel2C(H)=stackrel3C(H)stackrel4CH_3...
But "2-butylene" can generate geometric isomers, the which retain the SAME connectivity but a different geometry, viz. "cis" and "trans" isomers...
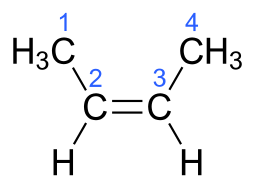 https://en.wikipedia.org/wiki/Butene
https://en.wikipedia.org/wiki/Butene
 https://en.wikipedia.org/wiki/Butene
https://en.wikipedia.org/wiki/Butene
In each case, the carbon-carbon connectivity is MANIFESTLY the same i.e. C1 connects to C2....to C4...but the geometry is manifestly different. And this gives rise to different physical properties, and chemical properties...i.e. the trans isomer has a boiling point of 0.9 ""^@C, whereas the cis isomer has a boiling point of 3.7 ""^@C.
That the trans isomer is MORE volatile than the cis isomer is an unusual phenomenon.
On the other hand, for "1-butylene" ONLY the one isomer is conceivable...
 commons.wikimedia.org
commons.wikimedia.org
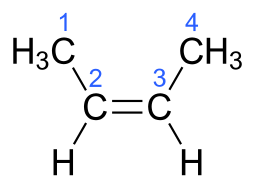 https://en.wikipedia.org/wiki/Butene
https://en.wikipedia.org/wiki/Butene  https://en.wikipedia.org/wiki/Butene
https://en.wikipedia.org/wiki/Butene  commons.wikimedia.org
commons.wikimedia.org 
