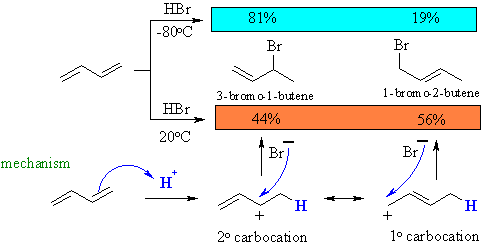
When 1,3 butadiene is treated with HBr in the presence of peroxides and light at very low temperatures two major products are produced how do you draw the 2 resonance structures for the intermediate radical and the final products?
1 Answer
First I'll describe what happens when HBr is added to a peroxide in the presence of light at low temp
Withut the catalyst the reaction would have the same products as dissociation of HBr also yields Br- but that would be slower
Sorry i cannot show the valence electrons because i cannot edit the letters so much
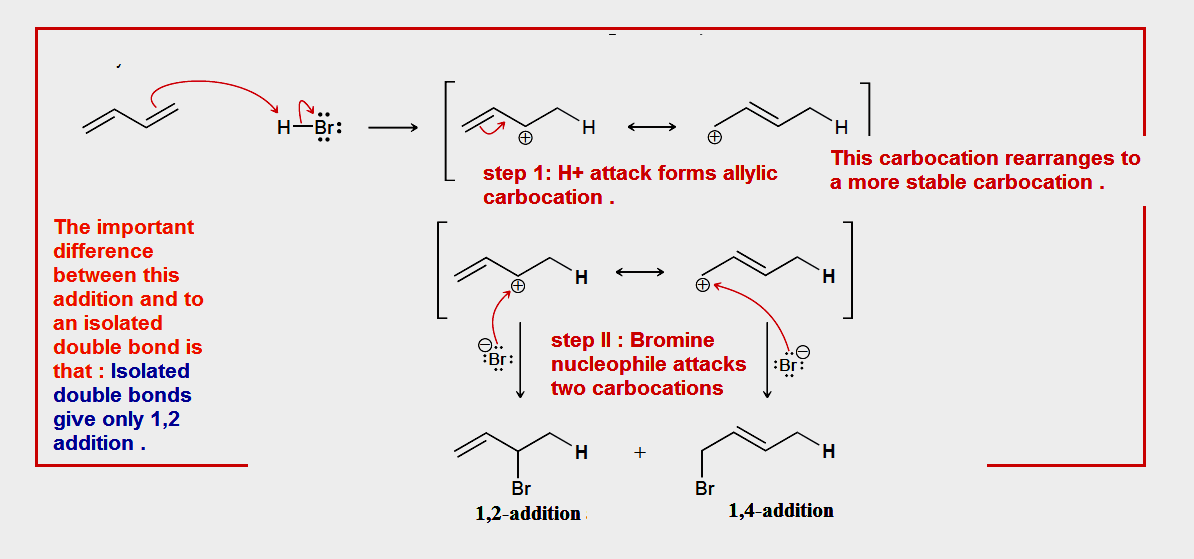
Because aromatic bonds donate electrons,
In intermediate (A) and (B) ,the aromatic bond will travel between the positive as the positive charge attracts electron density,but this would cause the original aromatic bond which is now no more there also positive . So the double bond would travel between the two carbon bonds
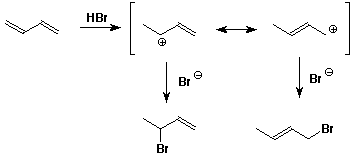
The final product is electron precise so no resonance will take place.Both of them do not have electrons sufficient for resonance

