Question #dcb61
1 Answer
Jan 29, 2018
The shape of s and p orbitals is determined by the angular momentum quantum number,
Explanation:
For d and f orbitals, the situation is not so simple, as these orbitals show different shapes, even though they possess the same value of
See the illustration:
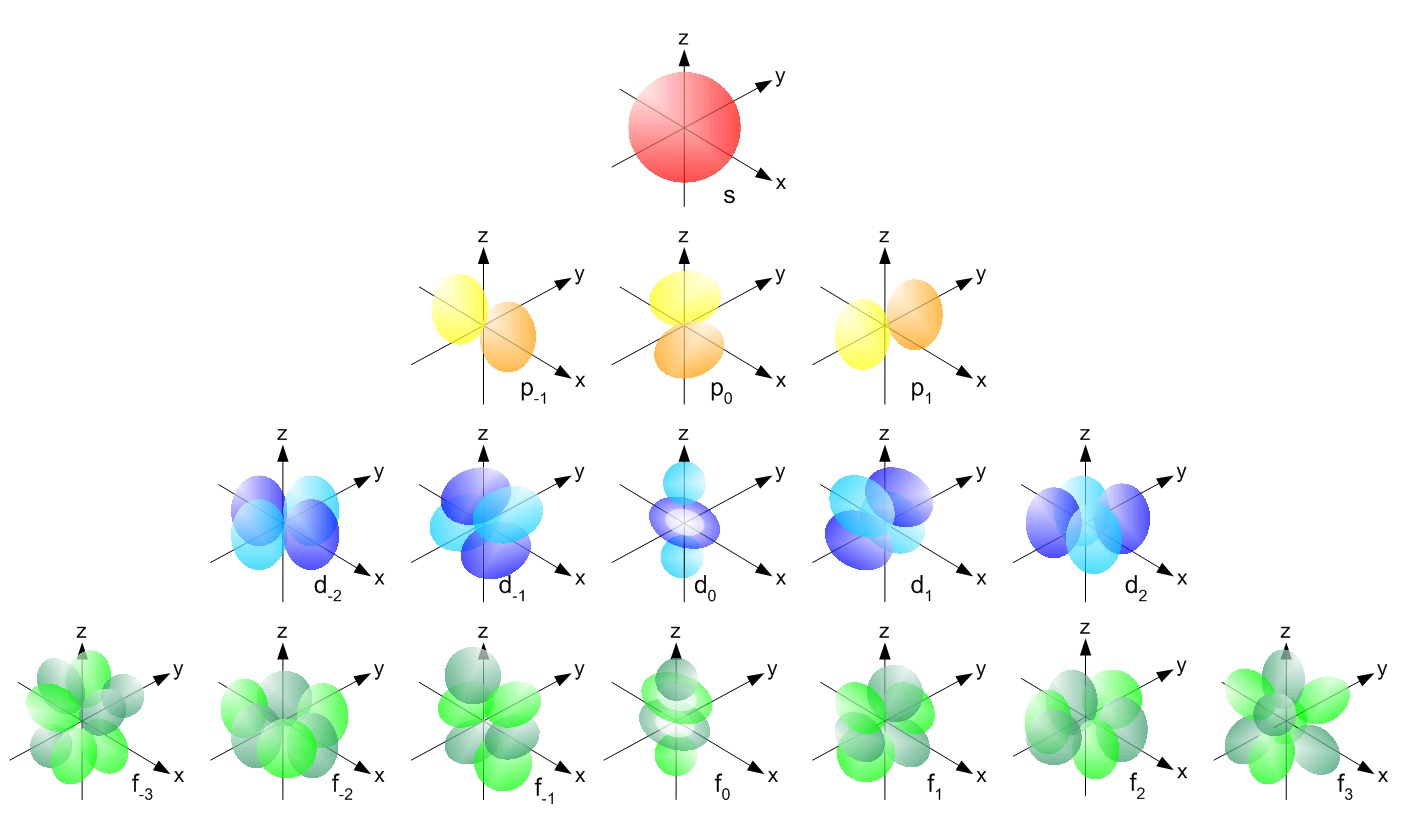
So, for the more complex cases, there is some dependence on the magnetic quantum number

