Question #c4172
1 Answer
Roman numerals are only used when a species with multiple charges is present in the reaction. Alkali metals only have one charge,
Explanation:
To be clear, roman numerals are only used in naming compounds whenever one of the species is available in different charges.
These species are more often than not transitional elements such as copper, lead, and iron.
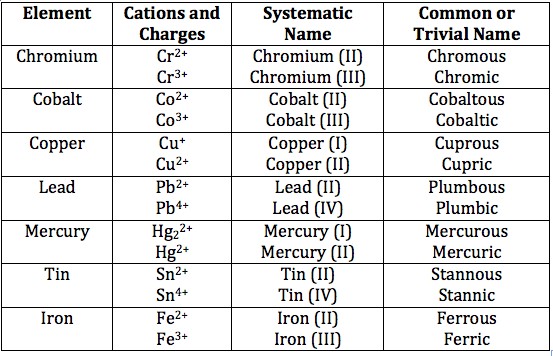
Roman numerals are then applied to indicate which species of an element is present in the reaction, and is written after the species name itself. E.g.
In the case with alkali metals, there is only one charge available for the element, and that is
Hope this helps :)

