Organic Chemistry Predict the product(s)?
1 Answer
1‐chloro‐1‐(prop‐1‐en‐2‐yl)cyclohexane and 1‐(2‐chloropropan‐2‐yl)cyclohex‐1‐ene
Explanation:
Double bonds are always a site of acid reactions so there are 2 double bonds, so two major reaction
If the
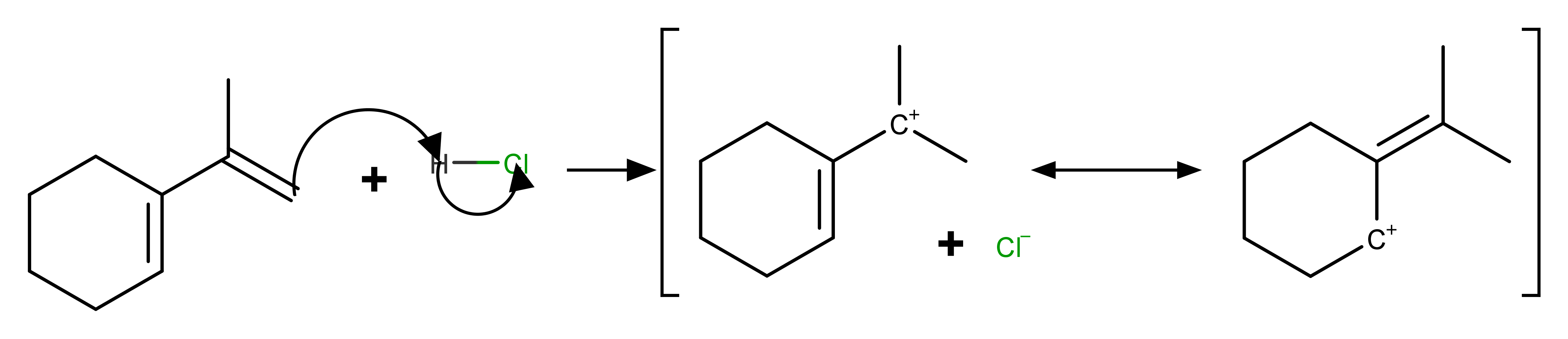
The proton does not gets added to the ring at the middle of the intersection of three bonds as the end of the side chain becomes positive and if this happens the intermediate is not resonance stabilized and even it is a primary carbocation so this reaction does not take place but if there is enough heat this compound may be formed in microscopic quantities
If the
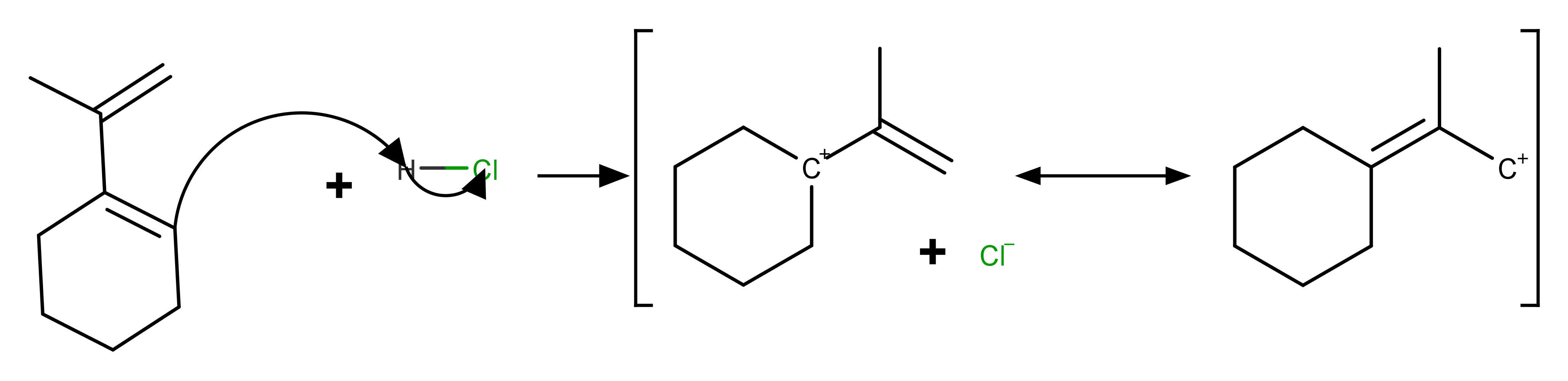
One is an unstable primary carbocation and another one is a tertiary carbocation.
I expect the 2 tertiary carbocations will be the creater of two of the major products
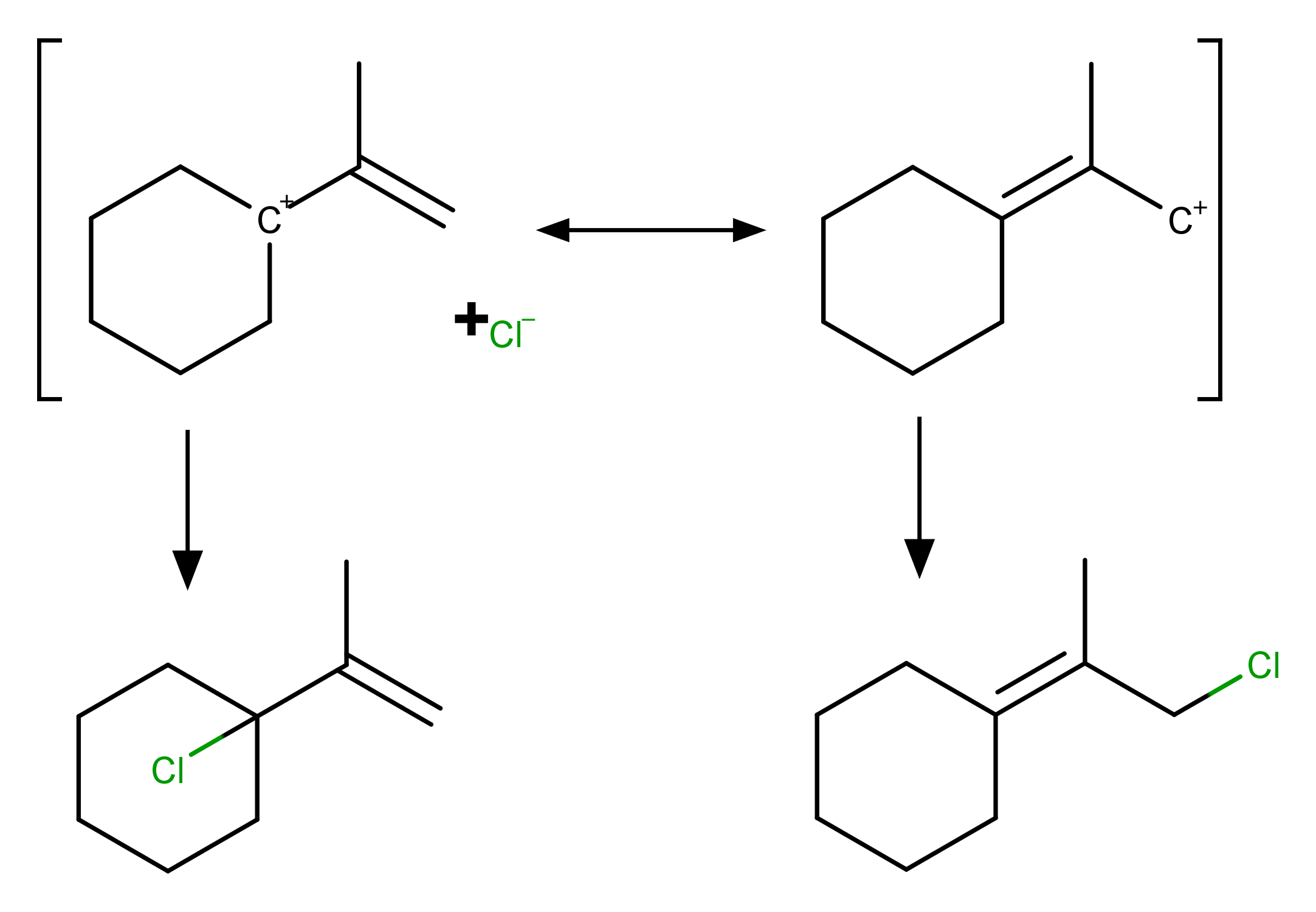
Here the major product is 1‐chloro‐1‐(prop‐1‐en‐2‐yl)cyclohexane
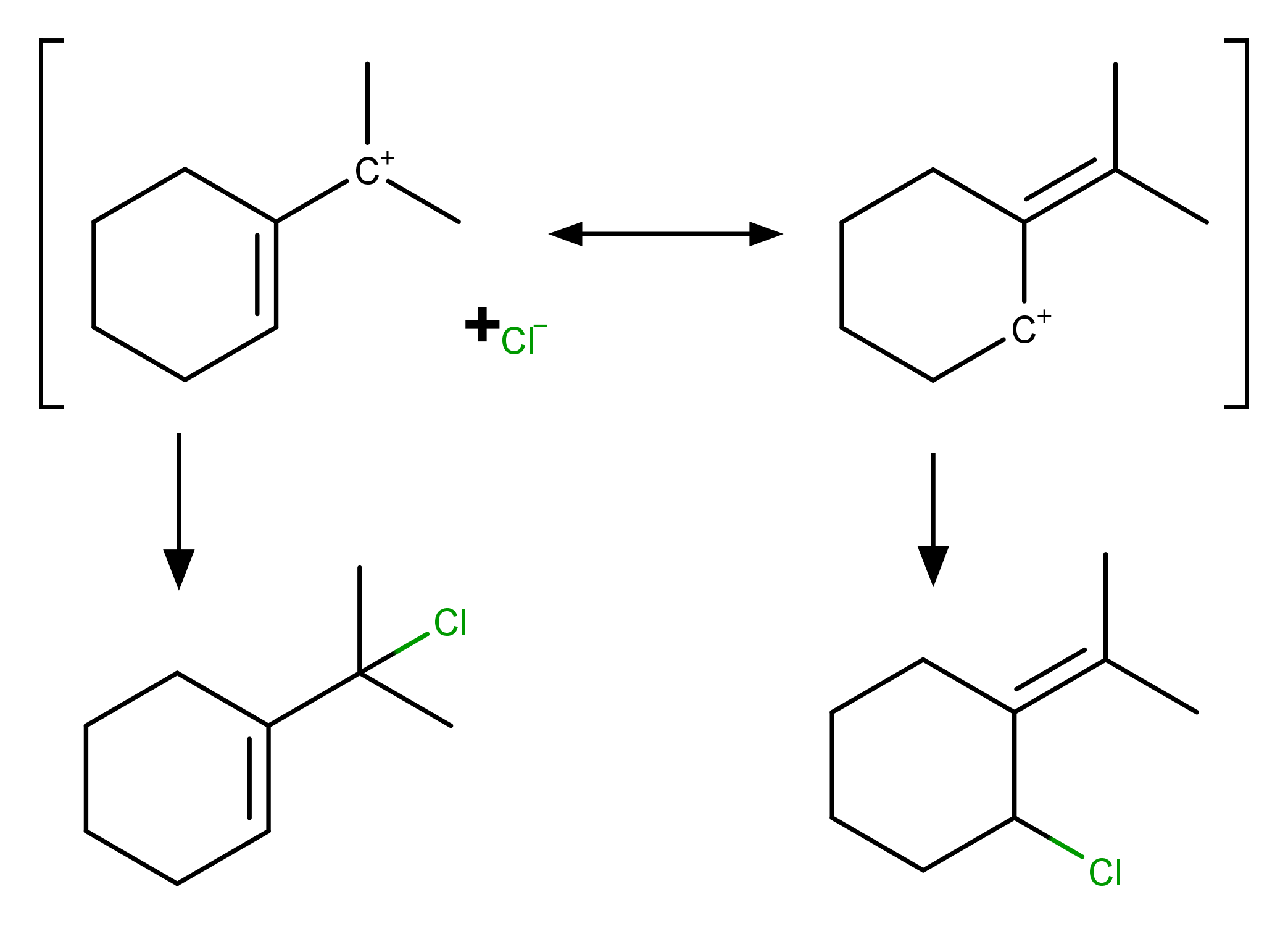
Here the major product is 1‐(2‐chloropropan‐2‐yl)cyclohex‐1‐ene

