Distinguish between the first, second and third ionization energies of an atom?
1 Answer
Well, by definition,
Explanation:
Well, by definition,
And
And the
And
And
From first principles, we would expect that
Well, for a start, the successive ionization energies remove an electron from an atom that is ALREADY positively charged. And, the electron may be a non-valence electron, i.e. it may be from an inner non-valence shell, for which more energy is required to remove this electron.
But a scientist interrogates data....
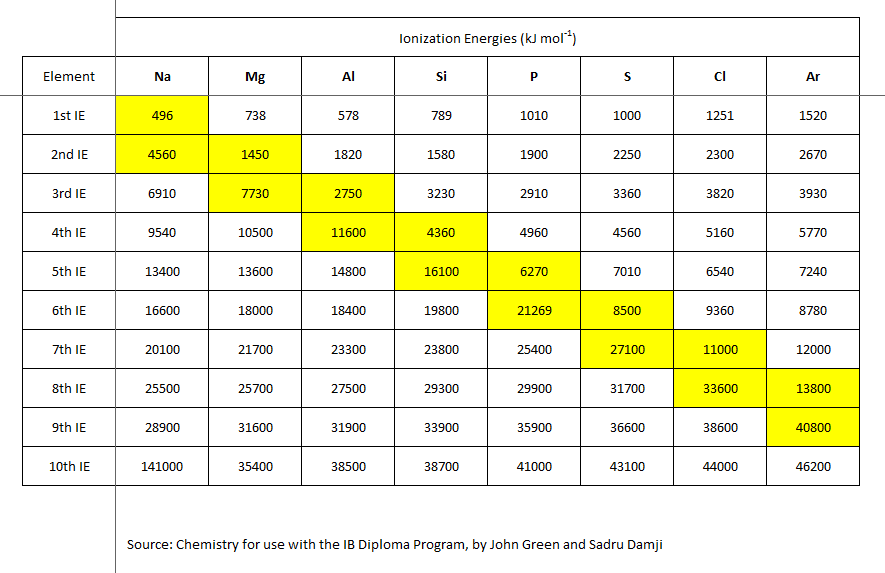
Are these data consistent with what we have argued?

